Cerebral Vision Impairment (CVI): Update on the Latest Diagnostic Tools, Techniques, and Treatment
Home / Pediatric Ophthalmology and Strabismus / Decreased Vision in Infants and Children
Title: Cerebral vision impairment (CVI): Update on the latest diagnostic tools, techniques, and treatment
Authors: Bryana Banashefski, MD, Terry L. Schwartz, MD, Sravanthi Vegunta, MD
Date: 02/02/2025
Keywords/Main Subjects: Cerebral vision loss, cortical blindness, cortical vision impairment, developmental delay, low vision, vision therapy
Description of Case: A definition and description of cerebral vision impairment (CVI) and various CVI evaluation methods
Summary of the Case: CVI is a leading causing vision impairment among children. It defined as visual dysfunction disorder with nonocular cause. Infants with hypoxic brain injury are at higher risk of developing CVI. Evaluation of visual acuity and visual processing may be difficult to assess in children with CVI using traditional visual acuity metrics in the clinic. Teachers of the visually impaired can work with clinicians to help determine visual potential in a child and direct therapies to improve visual processing.
Faculty Approval by: Griffin Jardine, MD
Introduction
Cerebral vision impairment (CVI) is a visual dysfunction disorder due to a non-ocular cause. The term cortical blindness was initially introduced in 1985.1 However, this term does not accurately encompass the anatomical localization of vision impairment in these patients.2 CVI is characterized by retro-geniculate visual pathway pathology that can develop perinatally. This leads to decreased bilateral visual acuity, visual field loss, and/or difficulties in higher order visuospatial processing.2–5 Ocular anatomy can be normal in these patients. If ocular pathology is also present, visual impairment is out of proportion to the pathology noted in patients. CVI is also distinct from vision impairment caused by acquired brain injury (e.g., trauma, stroke).2
In infants born at term (at or after 37 weeks gestational age), the most common cause of CVI is perinatal hypoxic-ischemic encephalopathy.6 In preterm infants, injury to the periventricular white matter, or periventricular leukomalacia (PVL), is the most common form of brain injury. The optic radiations, or the geniculocalcarine tract (e.g., Meyer’s loop, central bundle, and dorsal bundle), travel within the periventricular white matter (Figure 1), and therefore PVL is often associated with impaired visual processing or CVI.7,8
Teachers of the visually impaired (TVIs) along with primary care providers, occupational therapists, optometrists, neurologists, and ophthalmologists are all involved in diagnosing and managing CVI. It is important for ophthalmologists to understand the latest diagnostic techniques and treatments for CVI to appropriately counsel patients and their caregivers.
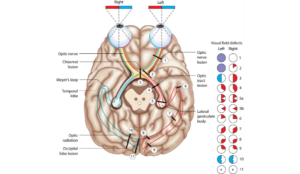
Figure 1. Reprinted with the approval of Dr. Daniel R. Gold. Reference URL: https://collections.lib.utah.edu/ark:/87278/s627k5w6
Diagnosing CVI
There are a wide variety of presenting symptoms in CVI. For example, the cortical ventral stream pathway takes visual information from the occipital lobe to the temporal lobe and facilitates recognition of objects and shapes (Figure 2). If a lesion is present in this pathway, patients could have challenges with object and face recognition. The dorsal stream pathway takes visual information from the occipital lobes to the parietal lobes and processes spatial recognition (Figure 2). A patient with a lesion in the dorsal stream pathway could present with trouble with spatial orientation.9 These higher order visual processing skills are difficult to identify and assess in the infant population and within the ophthalmology clinic alone.
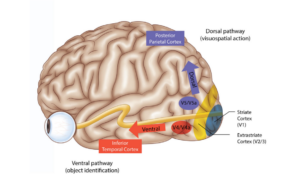
Figure 2. Reprinted with the approval of Dr. Daniel R. Gold. Reference URL: https://collections.lib.utah.edu/ark:/87278/s661xd3m
According to the Pediatric Cortical Visual Impairment Society,10 diagnostic criteria for CVI include
- The child must be at least 6 months of age.
- The vision loss is not explainable by abnormalities found on the eye examination.
- The child has a medical diagnosis that affects the brain.
- The child shows one of the following visual behaviors.
- Absent or clumsy visually guided motor response.
- Preferentially responds to a flashing light toy or brightly colored object (usually yellow or red) with ocular following or smiling or head/truncal movements.
- Responds more vigorously with visual stimulation approaching from one side of the visual field or the other.
- Has delayed responses to visual stimuli.
- Takes longer to fix and follow, or to re-fixate to target in peripheral vision.
- Looks up or away while looking at an object.
- Interacts visually in a more focused way when the workspace is uncluttered.
- Prefers to gaze at lights.
- Does not respond to people or large objects from across the room.
There is an overall lack of evidence and standardization for diagnosing CVI. Chang et. al and McConnell et al.’s reviews of visual assessment tools for children with CVI are summarized below.11,12 Often, patients will need to be referred to low vision clinics that are equipped with specialized tools for full diagnostic evaluation. TVIs complete these assessments.
Methods to Assess Visual Acuity
Visual behavior observation can be accomplished using various techniques including assessing interaction with objects. Patients with CVI may need larger targets.11 Additional visual behavior observations include the blink reflex, visual threat response, visual environmental exploration, visual fixation, visual attention, photophobia, and light perception.12
Forced-choice preferential looking (FPL) is a technique used to assess visual acuity in infants up to 5 months of age.13 Patients are presented with a card with a striped grating visual stimulus on one half and a reference (blank space) on the other. The examiner watches from behind the card through a peep hole in the card to see if the child preferentially looks toward the side of the stimulus as the stimulus alternates sides and increases in grating fineness (Figure 3). Visual acuity can be quantified by this method using grating acuity.
Sweep visual evoked potentials (VEP) is useful for patients who cannot participate in a subjective acuity test.14 The test measures electrical signals generated at the visual cortex in response to visual stimulation and determines the integrity of the visual pathways from the macula to the visual cortex. 33% of articles in McConnell et. al’s review used clinical electrophysiology to evaluate CVI patients.12 The limitation of the sweep visual evoked potentials test is that it cannot assess oculomotor abnormalities or higher level visual processing deficits.11
Methods to Assess Visual Parameters Other than Visual Acuity
Structured history-taking questionnaires can support the collection of critical history from parents and/or caregivers that can help diagnose or characterize CVI. Several symptoms of CVI are difficult to evaluate in the clinic alone due to the limited timeframe of appointments and the focus on evaluating patients for ocular pathology. McConnell et al. report that 37% of articles used structured history-taking questionnaires. The questionnaires listed below are validated screening questionnaires for the CVI population.
- Preverbal Visual Assessment (PreViA): Created to evaluate children less than two years old.15 The primary caregiver answers 30 questions related to visual domains: visual attention, visual communication, visual-motor coordination, and visual processing.
- Top-11 from the Higher Visual Function Question Inventory (Top-11)16: Consists of eleven questions that help to discriminate CVI behaviors from standard child behavior.
- Visual Skills Inventory (VSI)17: Includes 16 questions about visual skills and a child’s responses to familiar situations.
Eye Tracking is a noninvasive technique in which eye gaze is recorded while a subject views a visual stimulus on a computer monitor. A camera tracks the position of the corneal light reflex to monitor where the child is looking. A small, prospective study in 2021 evaluated the adaptation of eye tracking technology (SR Research EyeLink 1000 Plus) to assess CVI patient’s visual acuity.18
Visual perception testing:
- Children’s Visual Impairment Test for 3- to 6-year-olds (CVIT 3-6) measures visual perception impairments.19 The tests includes 14 subtests and takes under 25 minutes to complete, and it is freely available online.
- Beery-Buktenica Developmental Test of Visual-Motor Integration (VMI) assesses the extent to which individuals can integrate their visual and motor abilities.20 The test presents drawings that the patient is asked to copy. This test can be used for children ages 2-8 years old.
- The Austin Assessment is an iPad activity of matching cards showing different shapes. The patient uses their finger to move a card on top of another card when they believe they are a pair.21
- Lea® tests12
- Lea Mailbox® (Figure 4, B): The patient is asked to place a slip of paper through a horizontal opening. This test is used to assess spatial orientation.
- Lea Rectangles® (Figure 4, C): The patient is given rectangles of different sizes. The patient is asked to match the rectangles of the same size on top of one another. This test evaluates spatial recognition.
- Lea Heidi Expressions® (Figure 4, A): Can be used on children aged 2.5-3 years old. The patient is presented with two sets of drawings of six basic facial expressions, one expression on each card. The patient is asked to match the expressions with one another.
Neuroimaging was reported in 63% of the articles in McConnell et. al’s review, and the most common test was MRI brain.12 The relationship between imaging findings of visual pathway pathology, if present, and visual dysfunction in CVI remains poorly understood.22 Currently there is limited prognostic value in brain imaging regarding neurodevelopmental outcomes, but there are ongoing studies focused on understanding the relationship between the impact of injury and the trajectory of brain and vision development.22
Assessment Tools for standardizing diagnostic protocols and managing CVI include CVI Range and the ViBe matrix. CVI Range is an assessment tool composed of interviews, observations, and direct assessment that enables classification of CVI.4,23 It evaluates visual function on 10 characteristics, divided into three phases: Phase I (earliest visual functioning), Phase II (integration of vision with function), and Phase III (latest visual functioning). The 10 characteristics are preferred color, movement, latency, visual field, complexity, light-gazing, distance viewing, visual reflexes, novelty, and visually-guided reach. CVI Range uses a scale of 0-10 for each characteristic. A score of 0 indicates no visual response and a score of 10 represents typical vision.
Individuals in Phase I typically have intact dorsal stream only, which is responsible for spatial orientation. Patients in Phase I rarely look directly at an object and often have a strong color preference. Patients in Phase II can usually visually fixate, which reflects strengthening of the ventral stream. In Phase III, the goal is to improve ventral stream functioning. This is done by supporting patients in identifying visual details in complex visual settings.24
CVI Range lacks widespread adoption due to limited validation. There is currently an open research protocol aimed to assess the validity and reliability of CVI Range.25
CVI Treatment & Ophthalmologists Role
Treatment of CVI requires a multidisciplinary team approach, including pediatric ophthalmologists, neurologists, optometrists, occupational therapists, TVIs, and school teachers.27 To date, there is no evidence-based protocol for CVI rehabilitation. Given the wide range of symptoms and severity of CVI, individualized treatment plans must be generated for each patient. A scoping review published in 2023 synthesizes the current evidence for CVI treatment interventions.28 The treatments and scoping review findings are summarized below.
- Visual Stimulation: Eight studies included visual stimulation. These interventions use high contrast objects or environments to increase the child’s visual attention.
- Habilitations: Six studies included habilitations, or orientation and mobility training (O&M), which are recommendations and/or education on environmental modifications in the home or community settings to support the child’s visual processing and/or maximize the child’s function. Habilitations training teaches patients to use their remaining vision and other senses to get around. Canes and optical aids may also be used.29
- Color Tent: Two studies included color tent. This intervention eliminates visual clutter, presenting one uniform background to help build attentional capacity.
- Video Game: One study included video games to provide visual stimulation while challenging cognitive skills. These games can incorporate eye tracking data.
Conclusion
Pediatric ophthalmologists are usually the first providers to recognize a CVI suspect. CVI lacks standardized diagnostic procedures and robust, evidence-based treatment protocols. Given the wide variety of symptoms for CVI patients, individualized treatment plans curated through a multidisciplinary team are most common. It is important for ophthalmologists to understand the latest diagnostic tools to identify CVI early and setup their patients with a CVI care team for appropriate treatment.
CVI Resources
Perkins School for the Blind CVI Now website: https://www.perkins.org/cvi-now/
Dr. Terry Schwartz, Medical Director of Cincinnati Children’s Hospital Pediatric Low Vision Program, provided the following resources to support providers caring for patients with CVI.
- Template for history and physical exam
Loading...
- Sample Epic template for CVI evaluation
Loading...
- Mobile applications for low vision patients
Loading...
- Internet resources for CVI
Loading...
References
- Whiting S, Jan JE, Wong PKH, Flodmark O, Farrell K, McCormick AQ. PERMANENT CORTICAL VISUAL IMPAIRMENT IN CHILDREN. Dev Med Child Neurol. 1984;27(6):730-739. doi:10.1111/j.1469-8749.1985.tb03796.x
- Martín MBC, Santos-Lozano A, Martín-Hernández J, et al. Cerebral versus Ocular Visual Impairment: The Impact on Developmental Neuroplasticity. Front Psychol. 2016;7. doi:10.3389/fpsyg.2016.01958
- Cerebral Visual Impairment (CVI). NIH: National Eye Institute. Published July 14, 2020. Accessed September 20, 2023. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/cerebral-visual-impairment-cvi#:~:text=What%20is%20CVI%3F,but%20can%20continue%20into%20adulthood.
- Chang MY, Borchert MS. Advances in the evaluation and management of cortical/cerebral visual impairment in children. Surv Ophthalmol. 2020;65(6):708-724. doi:10.1016/j.survophthal.2020.03.001
- Hoyt CS. Visual function in the brain-damaged child. Eye. 2003;17(3):369-384. doi:10.1038/sj.eye.6700364
- Matsuba CA, Jan JE. Long-term outcome of children with cortical visual impairment. Dev Med Child Neurol. 2006;48(06):508. doi:10.1017/S0012162206001071
- Van Den Broeck C, Himpens E, Vanhaesebrouck P, Calders P, Oostra A. Influence of gestational age on the type of brain injury and neuromotor outcome in high-risk neonates. Eur J Pediatr. 2008;167(9):1005-1009. doi:10.1007/s00431-007-0629-2
- Hoyt CS. Brain injury and the eye. Eye. 2007;21(10):1285-1289. doi:10.1038/sj.eye.6702849
- Atkinson J. The Davida Teller Award Lecture, 2016: Visual Brain Development: A review of “Dorsal Stream Vulnerability”—motion, mathematics, amblyopia, actions, and attention. J Vis. 2017;17(3):26. doi:10.1167/17.3.26
- How Doctors Diagnose CVI. Pediatric Cortical Visual Impairment Society Accessed September 20, 2023. https://pcvis.vision/medical-professionals/how-doctors-diagnose-cvi/#:~:text=Professionals%20can%20make%20a%20diagnosis,diagnosis%20that%20affects%20the%20brain.
- Chang MY, Borchert MS. Methods of visual assessment in children with cortical visual impairment. Curr Opin Neurol. 2021;34(1):89-96. doi:10.1097/WCO.0000000000000877
- McConnell EL, Saunders KJ, Little J. What assessments are currently used to investigate and diagnose cerebral visual impairment (CVI) in children? A systematic review. Ophthalmic Physiol Opt. 2021;41(2):224-244. doi:10.1111/opo.12776
- Birch EE, Bane MC. FORCED-CHOICE PREFERENTIAL LOOKING ACUITY OF CHILDREN WITH CORTICAL VISUAL IMPAIRMENT. Dev Med Child Neurol. 2008;33(8):722-729. doi:10.1111/j.1469-8749.1991.tb14951.x
- Morelli F, Aprile G, Martolini C, et al. Visual Function and Neuropsychological Profile in Children with Cerebral Visual Impairment. Children. 2022;9(6):921. doi:10.3390/children9060921
- García-Ormaechea I, González I, Duplá M, Andres E, Pueyo V. Validation of the Preverbal Visual Assessment (PreViAs) questionnaire. Early Hum Dev. 2014;90(10):635-638. doi:10.1016/j.earlhumdev.2014.08.002
- Chandna A, Ghahghaei S, Foster S, Kumar R. Higher Visual Function Deficits in Children With Cerebral Visual Impairment and Good Visual Acuity. Front Hum Neurosci. 2021;15:711873. doi:10.3389/fnhum.2021.711873
- McCulloch DL, Mackie RT, Dutton GN, et al. A visual skills inventory for children with neurological impairments. Dev Med Child Neurol. 2007;49(10):757-763. doi:10.1111/j.1469-8749.2007.00757.x
- Chang MY, Borchert MS. Validity and reliability of eye tracking for visual acuity assessment in children with cortical visual impairment. J Am Assoc Pediatr Ophthalmol Strabismus. 2021;25(6):334.e1-334.e5. doi:10.1016/j.jaapos.2021.07.008
- Vancleef K, Janssens E, Petré Y, Wagemans J, Ortibus E. Assessment tool for visual perception deficits in cerebral visual impairment: reliability and validity. Dev Med Child Neurol. 2020;62(1):118-124. doi:10.1111/dmcn.14304
- Kulp MT, Sortor AJM. Clinical Value of the Beery Visual-Motor Integration Supplemental Tests of Visual Perception and Motor Coordination: Optom Vis Sci. 2003;80(4):312-315. doi:10.1097/00006324-200304000-00009
- McDowell N, Butler P. Validation of the Austin Assessment: A screening tool for cerebral visual impairment related visual issues. Satgunam P, ed. PLOS ONE. 2023;18(11):e0293904. doi:10.1371/journal.pone.0293904
- Merabet LB, Mayer DL, Bauer CM, Wright D, Kran BS. Disentangling How the Brain is “Wired” in Cortical (Cerebral) Visual Impairment. Semin Pediatr Neurol. 2017;24(2):83-91. doi:10.1016/j.spen.2017.04.005
- Roman-Lantzy C. Cortical Visual Impairment: An Approach to Assessment and Intervention. Second edition. AFB Press, American Foundation for the Blind; 2018.
- Baskin K. Getting Started with CVI Assessments. Perkins Sch Blind. https://www.perkins.org/getting-started-with-cvi-assessments/#:~:text=Christine%20Roman%2DLantzy’s%20CVI%20Range,sensory%20environment)%2C%20difficulty%20with%20distance
- Chang M, Roman-Lantzy C, O’Neil SH, Reid MW, Borchert MS. Validity and reliability of CVI Range assessment for Clinical Research (CVI Range-CR): a longitudinal cohort study. BMJ Open Ophthalmol. 2022;7(1):e001144. doi:10.1136/bmjophth-2022-001144
- Pilling RF, Allen L, Anketell P, Bullaj R, Harwood J, Little S. Visual Behaviours (ViBes) in Cerebral Visual Impairment: Validating a Descriptive Tool to Support Diagnosis and Monitoring. Br Ir Orthopt J. 2023;19(1):44-51. doi:10.22599/bioj.290
- Delay A, Rice M, Bush E, Harpster K. Interventions for children with cerebral visual impairment: A scoping review. Dev Med Child Neurol. 2023;65(4):469-478. doi:10.1111/dmcn.15431
- Weden K, DeCarlo DK, Barstow E. A Scoping Review of Intervention for Pediatric Cerebral Visual Impairment: Calling All Pediatric Occupational Therapists. Occup Ther Health Care. 2023;37(3):326-356. doi:10.1080/07380577.2023.2172761
- Virgili G, Rubin G. Orientation and mobility training for adults with low vision. Cochrane Eyes and Vision Group, ed. Cochrane Database Syst Rev. Published online May 12, 2010. doi:10.1002/14651858.CD003925.pub3
Copyright statement: Bryana Banashefski, Terry L. Schwartz, Sravanthi Vegunta, ©2025. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/





