A Case of Fulminant Serratia Marcescens Panophthalmitis after Penetrating Keratoplasty
Title: A Case of Fulminant Serratia Marcescens Panophthalmitis after Penetrating Keratoplasty
Authors: Meghan Sharma, MD, MPH1; Nour Bundogji, MD2; Nadim S. Azar, MD1; Amy Lin, MD1; Austin Nakatsuka, MD1
Affiliations:
1 John A. Moran Eye Center, University of Utah, Salt Lake City, UT, USA
2 John H. Stroger Jr. Hospital of Cook County, Chicago, IL, USA
Date: 6/24/2024
Keywords/Main Subjects: Fulminant panophthalmitis; Serratia marcescens panophthalmitis; penetrating keratoplasty; Serratia marcescens
Diagnosis: Fulminant Panophthalmitis
Description of Case: This is the first reported case of fulminant Serratia marcescens panophthalmitis after penetrating keratoplasty (PK).
Images:
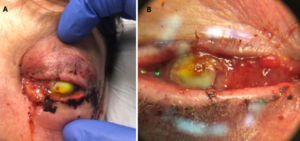
Figure 1A. External photograph of the patient’s left eye on post-operative day 2 following penetrating keratoplasty and secondary anterior chamber intraocular lens showing significant periorbital swelling, ecchymosis, and erythema as well purulent discharge and complete opacification of the cornea.
Figure 1B. Magnified photo of the patient’s left eye showing nasal chemosis, opacity of the PK graft, and diffuse, perforate corneal ulcer. PK graft appears to be in place and thickened and elevated nasally.
Note: The notable yellow tinge is fluorescein dye with complete uptake in the donor cornea.
Figure 2A. Histopathology consistent with an intense corneal ulcer at 20X magnification. The epithelium, Bowman’s, and endothelial cell layers are completely absent. Posteriorly, there are fragments of Descemet’s membrane showing folding and wrinkling.
Figure 2B. Histopathology at 200X magnification. The stroma shows a marked inflammatory cell reaction that is almost full thickness.
Figure 2C. Histopathology at 400X magnification. There is mixed inflammation consisting mostly of acute inflammatory cells with polymorphonuclear neutrophils, rare eosinophils, and occasional lymphocytes and plasma cells.
ABSTRACT
Purpose:
To report the first case of fulminant Serratia marcescens panophthalmitis after penetrating keratoplasty (PK).
Methods:
This is a report of a patient who developed fulminant panophthalmitis shortly after undergoing a PK with anterior chamber (AC) intraocular lens (IOL) placement. Slit-lamp examination as well as B-scan ultrasound (B scan) and orbital CT of the left eye (OS) were performed to further evaluate the patient. Tissue culture and histopathologic examination of the corneal specimen were completed to confirm the diagnosis.
Results:
A 78-year-old pseudophakic female presented with two days of increasing pain, swelling, and purulent discharge after uneventful PK and secondary AC-IOL placement OS. Examination was notable for light proception without projection, elevated intraocular pressure of 48 mmHg, and a perforated corneal ulcer. B scan demonstrated diffuse vitreous opacities and membranes. Orbital CT demonstrated proptosis and high attenuation material within the left globe. Canthotomy, vitreous sampling, and antibiotic injections were performed. Corneal tissue cultures grew S. marcescens. Therapeutic PK was performed, but after rapid decompensation, the eye was eviscerated.
Conclusion:
This is the only reported case of fulminant S. marcescens panophthalmitis after penetrating keratoplasty. S. marcescens panophthalmitis is an aggressive and rapidly progressive infection with poor visual outcomes despite appropriate intravitreal and systemic antibiotic therapy.
INTRODUCTION
Endophthalmitis, defined as inflammation of the inner structures of the eye with exudation in the vitreous cavity, is a rare but serious complication that may follow penetrating keratoplasty (PK). [1, 2] Endophthalmitis may progress to panophthalmitis, which involves the sclera and Tenon’s capsule, if the infection is not controlled promptly with antibiotics. [3] Progression of endophthalmitis to panophthalmitis following PK is extremely rare, with a 2018 study reporting that only 3.03% of panophthalmitis cases are post-PK. [2] Moreover, studies have often attributed post-PK endophthalmitis with gram-positive organisms as the most common cause of infection. [4] Only one case to our knowledge has been reported on endophthalmitis following PK due to Serratia marcescens, a gram-negative bacterium. [5] In this study, we report the first known case of S. marcescens fulminant panophthalmitis following PK.
MATERIALS AND METHODS
A search using the PubMed database was performed to identify relevant literature regarding post-PK endophthalmitis, post-PK panophthalmitis, S. marcescens endophthalmitis, and S. marcescens panophthalmitis. We report the first known case of fulminant S. marcescens panophthalmitis. We discuss the patient’s slit-lamp examination, B-scan ultrasound, and orbital computed tomography (CT) of the left eye (OS). Tissue culture and histopathologic examination of the corneal specimen confirming the diagnosis are also included in the report.
RESULTS
A 78-year-old pseudophakic female with a history of bilateral (OU) keratoconus status post PK and Descemet stripping endothelial keratoplasty presented with intraocular lens (IOL) dislocation and a seidel positive PK after slipping on wet leaves and falling on the ground. Routine cultures were not performed on the donor PK at the time. Eight months later, she underwent a secondary anterior chamber IOL procedure and repeat PK of the left eye (OS); however, she began experiencing increasing OS pain two days after the procedure. On post-operative day three, she presented to the emergency department with OS pain, redness, swelling, and purulent discharge (Figure 1). Ophthalmic exam demonstrated light perception (LP) visual acuity in the affected eye with an intraocular pressure (IOP) of 48. Slit lamp exam was notable for a diffuse, perforate corneal ulcer and no view to the anterior chamber or posterior pole but bloody vitreous. A B-scan ultrasound was performed, showing diffuse vitreous opacities and membranes. Orbital CT showed proptosis with high attenuation material within the left globe, likely representing hemorrhage with superimposed infection. Lateral canthotomy and cantholysis were performed in addition to a tap and injection of vancomycin with ceftazotide. Aerobic, viral, fungal, and anaerobic cultures were taken from the cornea during the tap. Following the procedure, the patient was empirically treated for panophthalmitis with IV vancomycin and piperacillin-tazobactam while being maintained on fortified antibiotics in the left eye, including vancomycin and tobramycin every one hour. Cultures of the corneal tissue grew 3+ gram-negative rods which were noted to be S. marcescens, sensitive to cefepime, ceftazidime, tobramycin, and fluoroquinolones.
The patient was diagnosed with fulminant S. marcescens panophthalmitis of the left eye, as evident from the ophthalmic exam, orbital CT, and bacterial cultures. Treatment was modified to include moxifloxacin 400 mg IV daily, cefepime 2g every 8 hours, and oral doxycycline to minimize corneal thinning. On post-operative day four, the patient underwent therapeutic PK, drainage of suprachoroidal hemorrhage, scleral patch graft, and repeat injections of the left eye. However, the patient continued to experience worsening pain despite treatment with oxycodone. The eye was firm to palpation with resistance to retropulsion, as intraocular and intraorbital pressure had increased. Slit lamp exam showed periorbital edema and ecchymoses with proptosis, disordered and atrophic conjunctiva with dried hemorrhage, a dark patch of likely necrotic sclera inferonasally that was seidel negative, and exposed sclera temporally and nasally. The PK graft was in place and seidel negative but was thickened and elevated nasally. Given the patient’s worsening pain, uncontrolled orbital pressure, and poor visual prognosis, evisceration OS was planned.
In addition to evisceration OS with an 18 mm silicone implant, the patient also underwent left temporary suture tarsorrhaphy and left lateral canthoplasty for fulminant S. marcescens panophthalmitis in a blind, painful eye. Subsequent histopathology (Figure 2) of the cornea was consistent with an intense corneal ulcer demonstrating complete absence of the epithelium, Bowman’s, and endothelial cell layers as well as an almost full thickness acute inflammatory cell reaction. Nine days after evisceration, the patient did not endorse any eye pain.
DISCUSSION
This is a rare case of a 78-year-old female with fulminant S. marcescens panophthalmitis following standard PK which resulted in evisceration of the eye. The literature regarding endophthalmitis or panophthalmitis following PK is limited. Of the literature reviewed, the most common pathogens in post-PK endophthalmitis are gram-positive bacteria. [4] One study analyzed 1,010 consecutive PKs and found three cases of bacterial endophthalmitis all caused by Streptococci with one case of Candida albicans endophthalmitis. [6] A 2003 review of 1,074 cases of endophthalmitis at Wills Eye Hospital identified ten cases of post-PK endophthalmitis that were due to gram-positive cocci (six Streptococcus species, three Staphylococcus species, and one identified on pathology specimen only) and only three cases were due to gram-negative organisms (Proteus mirabilis, Serratia marcescens, and one identified on pathology specimen only). The case of post-PK S. marcescens endophthalmitis in the Wills Eye Hospital study was resistant to ampicillin, bacitracin, and cefazolin and was only sensitive to ceftazidime. No specific information was found regarding treatment or prognosis in this patient. [5] This contrasts with our case of post-PK S. marcescens infection, which was sensitive to cefepime, ceftazidime, tobramycin, and fluoroquinolones. While the previous study details a case of S. marcescens endophthalmitis following PK, only three other cases of S. marcescens panophthalmitis have been found in literature. Unlike our case, these reported cases did not follow an ophthalmic surgery such as a PK, as the sources were endogenous. All patients in these cases reached LP vision or required evisceration or enucleation. [3, 7]
- marcescens is a gram-negative, opportunistic bacterium of the family Enterobacteriaceae and is the second most common pathogen among hospital-acquired ocular infections after Pseudomonas aeruginosa. [3] The species is typically found in water, soil, the gastrointestinal tract, and the urinary tract; however, our patient had no known predisposing factors for S. marcescens infection. [8] S. marcescens endophthalmitis may sometimes be observed as a pink hypopyon due to its distinctive red pigment, but this is not always seen, as in our patient. [8, 9] According to animal studies of S. marcescens ocular infection, the mechanism of action of S. marcescens appears to be a destructive process characterized by a hyperacute suppurative inflammatory response with liquefactive necrosis of ocular tissues thought to be mediated by the production of proteases. [10] S. marcescens endophthalmitis has been noted to have a poor prognosis. The largest reported series of patients with S. marcescens endophthalmitis noted that half (5/10) of patients had a final visual acuity of NLP or required evisceration, similar to the patient in this case report who required evisceration. [8]
Data on the treatment of S. marcescens endophthalmitis is limited. In the Endophthalmitis Vitrectomy Study, a multicenter randomized clinical trial examining systemic antibiotic treatment in the management of postoperative endophthalmitis, S. marcescens was not identified in any of the culture-positive isolates. [9] According to literature, empiric antibiotic treatment for post-PK endophthalmitis includes vancomycin to cover gram-positive bacteria and ceftazidime or gentamicin for gram-negative bacteria. [5] Fluoroquinolones have rapid bactericidal action by inhibiting bacterial DNA gyrase and topoisomerase IV and may be considered in the treatment of S. marcescens. In a 2007 in vitro study examining antibiotic effects and corneal epithelial toxicity of levofloxacin and moxifloxacin on human corneal epithelial cells, moxifloxacin was more effective for S. marcescens than levofloxacin; however, moxifloxacin seemed to exhibit more toxicity on human corneal epithelial cells at high concentrations and with long-term use. [11] S. marcescens may also demonstrate resistance to initial antibiotic therapy or require further therapy, as one study reported S. marcescens resistance to gentamicin as high as 90%. [9] In an institutional review of S. marcescens endophthalmitis cases at Bascom Palmer Eye Institute, 10% of patients demonstrated persistent growth of S. marcescens on repeat vitreous cultures, and several others had further clinical deterioration despite use of sensitive antibiotics. [9] Similarly, our patient in this case report was sensitive to several antibiotics while on moxifloxacin and cefepime; however, her pain continued to worsen with no improvement in vision, ultimately leading to evisceration. In conclusion, this case study emphasizes that S. marcescens panophthalmitis is an aggressive and rapidly progressive infection with poor visual outcomes despite appropriate intravitreal and systemic antibiotic therapy.
References:
- Chen JY, Jones MN, Srinivasan S, et al., Endophthalmitis after penetrating keratoplasty. Ophthalmology, 2015. 122(1): p. 25-30.
- Pappuru RR, Dave VP, Pathengay A, et al., Endophthalmitis Progressing to Panophthalmitis: Clinical Features, Demographic Profile, and Factors Predicting Outcome. Semin Ophthalmol, 2018. 33(5): p. 671-674.
- Guo HP, Wang TJ, Fulminant Serratia marcescens Panophthalmitis. Am J Med Sci, 2017. 353(4): p. e7.
- Alharbi SS, Alrajhi A, Alkahtani E, Endophthalmitis following keratoplasty: incidence, microbial profile, visual and structural outcomes. Ocul Immunol Inflamm, 2014. 22(3): p. 218-23.
- Kunimoto DY, Tasman W, Rapuano C, et al., Endophthalmitis after penetrating keratoplasty: microbiologic spectrum and susceptibility of isolates. Am J Ophthalmol, 2004. 137(2): p. 343-5.
- Kloess PM, Stulting RD, Waring GO, et al., Bacterial and fungal endophthalmitis after penetrating keratoplasty. Am J Ophthalmol, 1993. 115(3): p. 309-16.
- Breazzano MP, Jonna G, Nathan NR, et al., Endogenous Serratia marcescens panophthalmitis: A case series. Am J Ophthalmol Case Rep, 2019. 16: p. 100531.
- Goldenberg DT, Harinandan A, Walsh MK, et al., Serratia marcescens endophthalmitis after 20-gauge pars plana vitrectomy. Retin Cases Brief Rep, 2010. 4(2): p. 140-2.
- Bhikoo R, Blakiston M, Cunningham W, et al., Serratia Marcescens Endophthalmitis and Bacteraemia following Complicated Cataract Surgery. Ocul Immunol Inflamm, 2022. 30(4): p. 1020-1021.
- Sridhar J, Kuriyan AE, Flynn Jr. HW, et al., ENDOPHTHALMITIS CAUSED BY SERRATIA MARCESCENS: Clinical Features, Antibiotic Susceptibilities, and Treatment Outcomes. Retina, 2015. 35(6): p. 1095-100.
- Kim SY, Lim JA, Choi JS, et al., Comparison of antibiotic effect and corneal epithelial toxicity of levofloxacin and moxifloxacin in vitro. Cornea, 2007. 26(6): p. 720-5.
Faculty Approval by: Dr. Austin Nakatsuka
Copyright Statement: Copyright Meghan Sharma, Nour Bundogji, Nadim S. Azar, Amy Lin, Austin Nakatsuka, ©2024. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/