Pentosan maculopathy
Title: Pentosan maculopathy
Author (s): Ivan Cardenas, BS, Nnana Amakiri, MD, Akbar Shakoor, MD, Eileen Hwang, MD, PhD
Date: 10/23/2023
Keywords/Main Subjects: Pentosan maculopathy, AMD, drug toxicity, pentosan polysulfate sodium, interstitial cystitis
Diagnosis: Pentosan Maculopathy
Case Report:
This is a case of a 68-year-old woman with a three-year history of progressing drusen, diagnosed with age-related macular degeneration (AMD) by an outside ophthalmology office. She was referred to the Moran Eye Center for further workup due to the persistent progression of her findings, and an increasing suspicion of medication toxicity. She had a history of interstitial cystitis diagnosed approximately 27 years prior to her presentation, for which she started treatment with pentosan polysulfate sodium (pentosan). She did not have any other ocular conditions or surgeries and no family history of ocular disease. Other than her interstitial cystitis, she had a history of muscular dystrophy, hypertension, hyperlipidemia, and myocardial infarction. Besides pentosan, she was taking AREDS vitamins, estradiol, gabapentin, and amitriptyline.
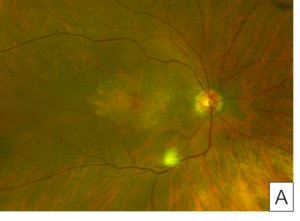
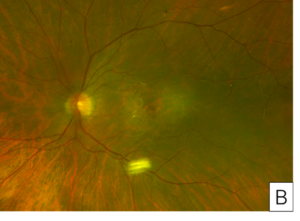
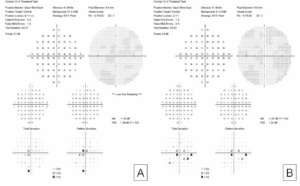
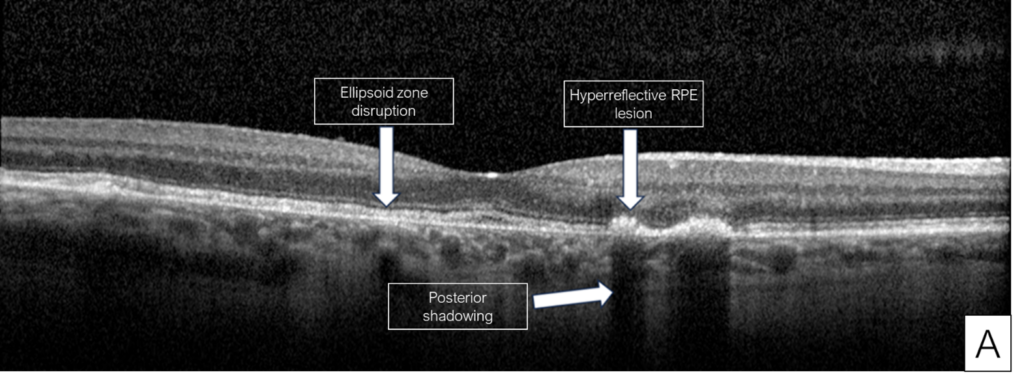
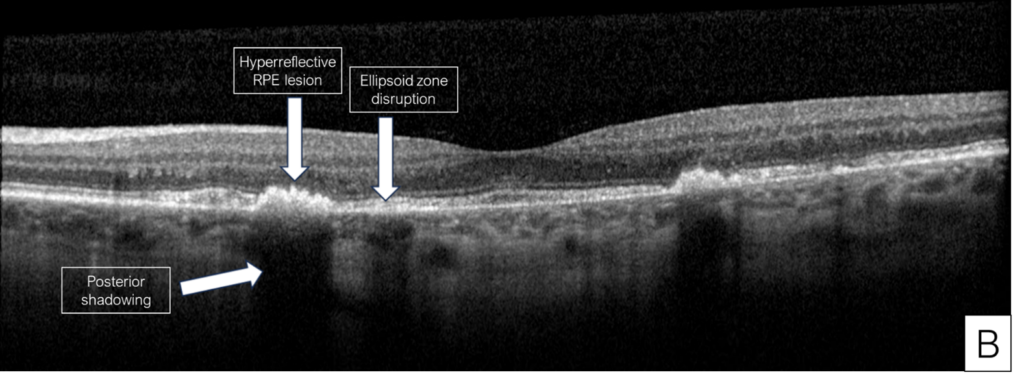
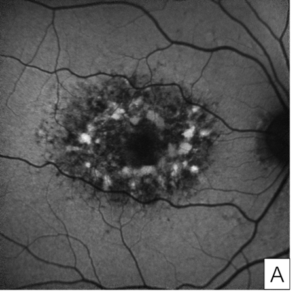
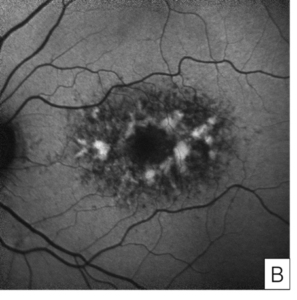
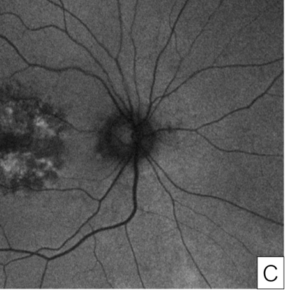
Her exam demonstrated a visual acuity of 20/30 in both eyes, physiologic intraocular pressure, and a slit lamp exam without significant abnormalities. Dilated fundus exam and color fundus imaging demonstrated hypopigmentation of the retinal pigment epithelium in the central macula and mild peripapillary atrophy bilaterally (Figure 1). Humphrey visual field showed a paracentral defect in the left eye and central defect in the right eye (Figure 2). On optical coherence tomography (OCT), there was ellipsoid zone (EZ) disruption bilaterally, with multifocal hyperreflective retinal pigment epithelial (RPE) lesions (Figure 3). On fundus autofluorescence imaging, the macula showed a densely packed array of hyperautofluorescent and hypoautofluorescent spots (Figure 4A and 4B) and the optic nerve showed a ring of hypoautofluorescence, which correlated with the peripapillary atrophy seen on color fundus imaging (Figure 4C and 4D). Infrared imaging of the macula showed hyperreflective punctate lesions scattered throughout the macula (Figure 5).
She lacked the classic findings seen in AMD or pattern dystrophies, and due to the unique characteristics found in her workup in the setting of possible medication toxicity, she was diagnosed with pentosan maculopathy. After informed consent and shared decision making, the decision was made to cease the medication. She continues to be followed for evidence of progression, given the possibility of disease progression after cessation of the drug. She also has remained under close care by her urologist, due to worsening of the pain associated with her interstitial cystitis.
Discussion:
Pentosan maculopathy is a rare ocular condition caused by use of pentosan polysulfate sodium, a drug approved by the FDA in 1996 for the treatment of interstitial cystitis. Ocular toxicity of pentosan was first described in a 6-patient cohort in 2018.1 One study found ocular toxicity in 20% of patients who had a cumulative dose of pentosan greater than 500g (equivalent to 4.5 years at the standard dose).2 Pentosan toxicity preferentially impacts the macula and is believed to be a result of cumulative exposure to the drug.3,4,5 It commonly presents with blurred vision, difficulty with dim lighting and/or dark adaptation, and difficulty reading, though visual acuity is generally preserved in early maculopathy. While the exact mechanism of its impact on the retina is unknown, ongoing case reports and studies in mice suggest possible disruption of the glycosaminoglycans in the RPE-photoreceptor interface, as well as possible fibroblast growth factor signal inhibition or choriocapillaris injury.6,7,8,9
The patient described here was initially misdiagnosed with dry AMD, which highlights the importance of asking AMD patients about their medical history, and particularly their medications. Without this information, the etiology can easily be missed and may lead to progression of the condition. While this patient’s findings were consistent with pentosan maculopathy, different forms of maculopathies were considered, such as AMD with geographic atrophy, hereditary maculopathies, or pattern dystrophies. The diagnosis of pentosan maculopathy was reinforced through distinct imaging characteristics seen on OCT and fundus autofluorescence. The OCT of our patient showed multifocal hyperreflective retinal pigment epithelial lesions with posterior shadowing (Figure 3) characteristic of pentosan maculopathy. These lesions are distinct from the drusen seen in dry AMD, which are less hyperreflective, and located under the retinal pigment epithelium rather than intrinsic to the retinal pigment epithelium (Figure 6).3 The autofluorescence images of our patient demonstrate a densely packed array of hyperautofluorescent and hypoautofluorescent spots involving the posterior pole (Figure 4).3 While pattern dystrophies may also be characterized by hyper- and hypoautofluorescent patches, lines and spots (Figure 7),10,11,12 the pattern of autofluorescence in pentosan maculopathy is more densely clustered (Figure 4).
Beyond OCT and autofluorescence, near infrared imaging has also been used as an imaging modality for the disease, where it has been proposed for mild cases where it demonstrates parafoveal hyperreflective punctate lesions as was seen in our case (Figure 5).3,13 Advanced pentosan maculopathy may result in retinal pigment epithelium atrophy (Figure 8), although our case was early in its course without atrophy.4,9 In one study, RPE atrophy which extended into the fovea was used as a criteria for severe pentosan maculopathy, and was associated with very poor visual acuity.9 Studies have also reported peripapillary halos1,3,9,13,, defined as a circumferential ring of hypoautofluorescence surrounding the optic nerve in the absence of peripapillary atrophy. While our case did demonstrate a ring of hypoautofluorescence, it was consistent with the peripapillary atrophy seen on color fundus imaging so was not described as a halo. Peripapillary atrophy typically results in hypoautofluorescence surrounding the optic nerve14, but the atrophy can be distinguished from the halo by hypopigmentation on color fundus photos or examination.15,16 These peripapillary halos have been noted even in mild cases, and are not typically seen in other retinal conditions.3,9 Taken together, this constellation of findings is unique to pentosan maculopathy and provided us with the necessary evidence to diagnose the condition.
Many experts agree on the importance of comprehensive eye exams to screen patients for signs of pentosan maculopathy. Research publications emphasize that these exams should include multimodal imaging, especially autofluorescence,1,4,17 given the high specificity of autofluorescence findings. Conflicting guidelines exist for when screening exams should begin. Some researchers have recommended beginning screening eye exams after a cumulative dose of 500g.3 On the other hand, the American Urogynecologic Society has recommended baseline evaluation followed by annual screening starting after five years,18 and the manufacturer of Elmiron, the brand name for pentosan, recommends baseline evaluation within 6 months of initiation and periodic evaluation.19 While stopping this drug gives the best chance of vision preservation, it may worsen the interstitial cystitis as was seen in our patient’s case. It may also continue to progress after cessation, although there are no current estimates on how long the progression may continue.20,21 When addressing toxicities and systemic illnesses impacting the retina, it is imperative to engage in multidisciplinary discussions among healthcare providers and actively involve patients in shared decision-making to ascertain the most optimal treatment approach.
Figures
Figure 1. Color fundus images of right eye (A) and left eye (B) showing hypopigmentation of the retinal pigment epithelium in the central macula and mild peripapillary atrophy bilaterally.
Figure 2. Central 10-2 Humphrey Visual Field showing central defects in the right eye (A) and paracentral defects in the left eye (B).
Figure 3. Optical coherence tomography (OCT) of the right eye (A) and left eye (B) showing features characteristic of early pentosan maculopathy.
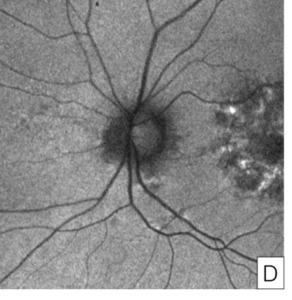
Figure 4. Fundus autofluorescence of the macula in the right eye (A) and left eye (B) showing characteristic features of pentosan maculopathy, specifically densely packed array of hyperautofluorescent and hypoautofluorescent spots. Autofluorescence of the optic nerve of the right eye (C) and left eye (D) showing a circumferential ring of hypoautofluorescence consistent with areas of peripapillary atrophy seen on color fundus imaging.
Figure 5. Near infrared reflectance imaging of the right eye (A) and left eye (B) showing hyperreflective punctate lesions scattered throughout macula.
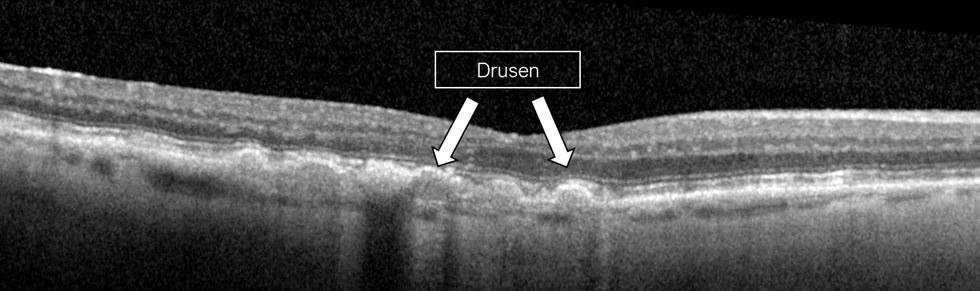
Figure 6. OCT findings of intermediate dry AMD showing drusen, which are hyperreflective deposits beneath the retinal pigment epithelium.
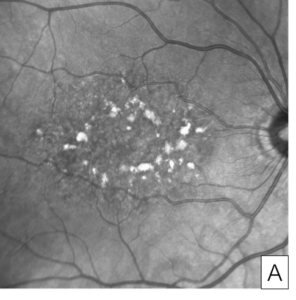
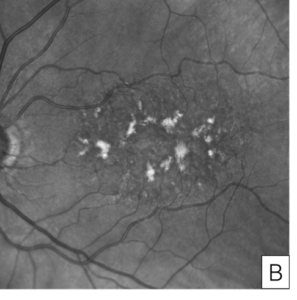
Figure 7. Autofluorescence image of butterfly pattern dystrophy showing linear patterns of hyperautofluorescence on a hypoautofluorescent background (A). Autofluorescence image of adult foveomacular vitelliform dystrophy showing a round hyperautofluorescent spot within the fovea (B).
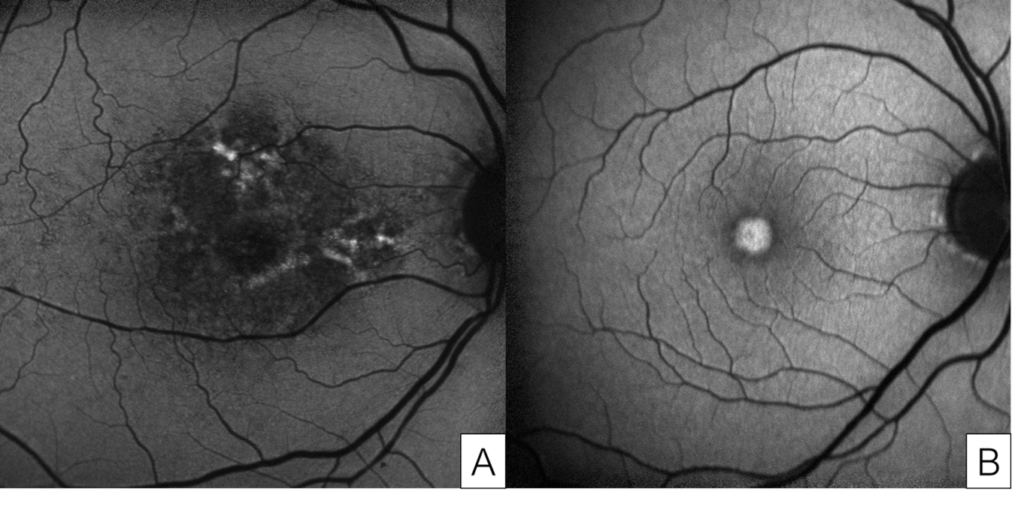
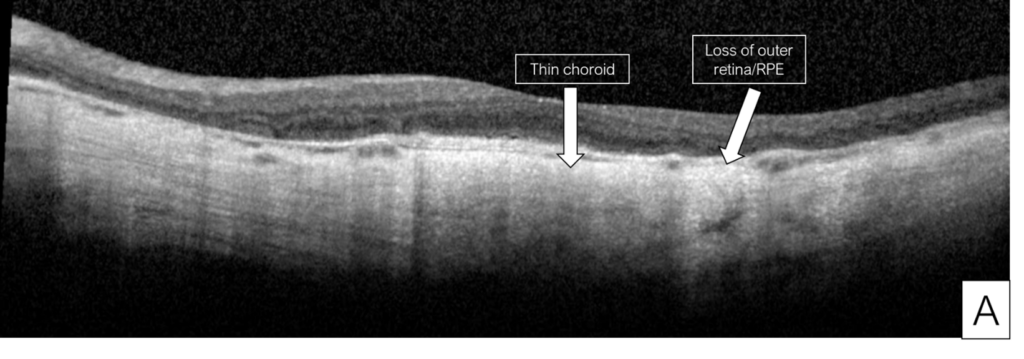
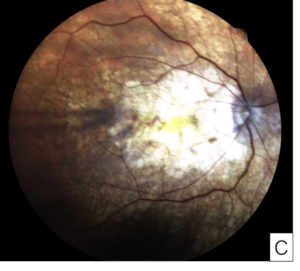
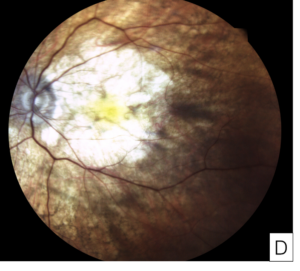
Figure 8. Images from a patient with severe pentosan maculopathy and count fingers vision in both eyes due to retinal pigment epithelium atrophy involving the fovea. Optical coherence tomography shows loss of the outer retina and retinal pigment epithelium, and thin choroid (A, B). Color fundus photographs show that the hypopigmentation due to atrophy involves most of the macula (C, D).
References
- William A. Pearce, Rui Chen, Nieraj Jain. Pigmentary Maculopathy Associated with Chronic Exposure to Pentosan Polysulfate Sodium. Ophthalmology, Volume 125, Issue 11, 2018, Pages 1793-1802, ISSN 0161-6420, https://doi.org/10.1016/j.ophtha.2018.04.026.
- Vora, Robin A. et al. Prevalence of Maculopathy Associated with Long-Term Pentosan Polysulfate Therapy. Ophthalmology, Volume 127, Issue 6, 835 – 836
- Wang D, Velaga SB, Grondin C, Au A, Nittala M, Chhablani J, Vupparaboina KK, Gunnemann F, Jung J, Kim JH, Ip M, Sadda S, Sarraf D. Pentosan Polysulfate Maculopathy: Prevalence, Spectrum of Disease, and Choroidal Imaging Analysis Based on Prospective Screening. Am J Ophthalmol. 2021 Jul;227:125-138. doi: 10.1016/j.ajo.2021.02.025. Epub 2021 Feb 27. PMID: 33651989.
- Kalbag NS, Maganti N, Lyon AT, Mirza RG. Maculopathy Secondary to Pentosan Polysulfate Use: A Single-Center Experience. Clin Ophthalmol. 2021 Feb 11;15:513-519. doi: 10.2147/OPTH.S285013. PMID: 33603329; PMCID: PMC7884940.
- Garzon S, Laganà AS, Casarin J, Raffaelli R, Cromi A, Sturla D, Franchi M, Ghezzi F. An update on treatment options for interstitial cystitis. Prz Menopauzalny. 2020 Mar;19(1):35-43. doi: 10.5114/pm.2020.95334. Epub 2020 Apr 27. PMID: 32699542; PMCID: PMC7258371.
- Preston Girardot, Xian Zhang, Isabelle Gefke, Wenfei Wu, Adam Marcus Hanif, Nieraj Jain, John M Nickerson, Jeffrey H Boatright; Systemic Pentosan Polysulfate Administration Causes Retinal Function Loss in the C57Bl/6J Mouse. Ophthalmol. Vis. Sci.2019;60(9):2352.
- Preston E. Girardot, Xian Zhang, Nan Zhang, Kevin J. Donaldson, Micah Chrenek, Jana T Sellers, Nieraj Jain, John M Nickerson, Jeffrey H Boatright; Pentosan Polysulfate Retinopathy in Mice Involves Subtle Changes in Outer Segment Length and RPE Cell Size. Ophthalmol. Vis. Sci.2022;63(7):1903 – A0049.
- Abou-Jaoude, Michelle M., et al. New Insights Into Pentosan Polysulfate Maculopathy. Ophthalmic Surgery, Lasers, and Imaging Retina, vol. 52, no. 1, Jan. 2021. https://doi.org/10.3928/23258160-20201223-04
- Hanif AM, Armenti ST, Taylor SC, Shah RA, Igelman AD, Jayasundera KT, Pennesi ME, Khurana RN, Foote JE, O’Keefe GA, Yang P, Hubbard GB 3rd, Hwang TS, Flaxel CJ, Stein JD, Yan J, Jain N. Phenotypic Spectrum of Pentosan Polysulfate Sodium-Associated Maculopathy: A Multicenter Study. JAMA Ophthalmol. 2019 Nov 1;137(11):1275-1282. doi: 10.1001/jamaophthalmol.2019.3392. PMID: 31486843; PMCID: PMC6735406.
- Antonelli, G.; Parravano, M.; Barbano, L.; Costanzo, E.; Bertelli, M.; Medori, M.C.; Parisi, V.; Ziccardi, L. Multimodal Study of PRPH2 Gene-Related Retinal Phenotypes. Diagnostics 2022, 12, 1851. https://doi.org/10.3390/diagnostics12081851
- Chowers I, Tiosano L, Audo I, Grunin M, Boon CJ. Adult-onset foveomacular vitelliform dystrophy: A fresh perspective. Prog Retin Eye Res. 2015 Jul;47:64-85. doi: 10.1016/j.preteyeres.2015.02.001. Epub 2015 Feb 11. PMID: 25681578.
- Tiosano L, Grunin M, Hagbi-Levi S, Banin E, Averbukh E, Chowers I. Characterising the phenotype and progression of sporadic adult-onset foveomacular vitelliform dystrophy. Br J Ophthalmol. 2016 Nov;100(11):1476-1481. doi: 10.1136/bjophthalmol-2015-307658. Epub 2016 Jan 22. PMID: 26802173.
- Aaron Lindeke-Myers, Adam M. Hanif, Nieraj Jain. Pentosan polysulfate maculopathy. Survey of Ophthalmology, Volume 67, Issue 1, 2022, Pages 83-96, ISSN 0039-6257, https://doi.org/10.1016/j.survophthal.2021.05.005.
- Sayed SY, Raafat KA, Ahmed RA, Allam RSHM. Evaluation of peripapillary atrophy in early open-angle glaucoma using autofluorescence combined with optical coherence tomography. Int Ophthalmol. 2021 Jul;41(7):2405-2415. doi: 10.1007/s10792-021-01795-0. Epub 2021 Apr 29. PMID: 33928472.
- Leung EH, Sharma S, Levie-Sprick A, Lee GD, Cho H, Mukkamala K. Pentosan Polysulfate Sodium-Associated Pigmentary Retinopathy: Risk Factors and Fundus Findings. Clin Ophthalmol. 2021 Dec 24;15:4809-4816. doi: 10.2147/OPTH.S340041. PMID: 34992341; PMCID: PMC8714003.
- Khodeiry MM, Liu X, Sayed MS, Goldhardt R, Gregori G, Albini TA, Lee RK. Peripapillary Halo in Inflammatory Papillitis of Birdshot Chorioretinopathy. Clin Ophthalmol. 2021 Jun 3;15:2327-2333. doi: 10.2147/OPTH.S307589. PMID: 34113076; PMCID: PMC8184253.
- Nieraj Jain, Albert Liao, Sunir J. Garg, Samir N. Patel, Charles C. Wykoff, Hannah J. Yu, Nikolas J.S. London, Rahul N. Khurana, David N. Zacks, Macula Society Pentosan Polysulfate Maculopathy Study Group. Expanded Clinical Spectrum of Pentosan Polysulfate Maculopathy: A Macula Society Collaborative Study, Ophthalmology Retina, Volume 6, Issue 3, 2022, Pages 219-227, ISSN 2468-6530, https://doi.org/10.1016/j.oret.2021.07.004.
- Practice Advisory: Possible drug induced retinal maculopathy secondary to long-term use of pentosan polysulfate sodium (Elmiron). American Urogynecologic Society. Published August 2020. Accessed February 26, 2021.
- Janssen (n.d.). Elmiron (pentosan polysulfate sodium) capsules [Prescribing information]. Orthoelmiron. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/ELMIRON-pi.pdf
- Guo LY, Mishra K, Leung LB. Severe progression of pentosan maculopathy over a decade. Am J Ophthalmol Case Rep. 2023 Mar 8;30:101826. doi: 10.1016/j.ajoc.2023.101826. PMID: 36941944; PMCID: PMC10023981.
- Shah R, Simonett JM, Lyons RJ, Rao RC, Pennesi ME, Jain N. Disease Course in Patients With Pentosan Polysulfate Sodium–Associated Maculopathy After Drug Cessation. JAMA Ophthalmol. 2020;138(8):894–900. doi:10.1001/jamaophthalmol.2020.2349