Ciliopathy
Title: Ciliopathy
Author (s): Tanner Nelson, MSIV
Date: July 29, 2024
Diagnosis: Hereditary Ciliopathy, IFT 140 mutation
Description of Case: Patient is an 8-year old female with a history of IFT-140 gene mutation who presents to clinic. VA is approximately 20/400 OU. Patient has concomitant nephropathy characterized by mildly enlarged, echogenic kidneys, as well as skeletal abnormalities, including facial dysmorphism and hip dysplasia. On OCT, the patient demonstrates a bulls-eye maculopathy with significant outer retinal drop, including loss of the IS/OS junction of the parafoveal area. The patient requires large- print to read and uses a cane to walk, especially in dim light.
Background:
Ciliopathy refers to disease of cilia, a microscopic organelle associated with most cells of the body. Cilia can be motile, as can be found in the airway and other organs, or non-motile. The major category of non-motile cilia is called primary cilia, which plays an important role in many cellular processes, such as transport and signaling.1,2 Primary cilia are found in many cells of the body, including the retina, kidney, musculoskeletal system, and many other organs. Therefore, patients with a defect in a gene responsible for structure or function of the primary cilia often have profound defects of these organs.3
There are many syndromes caused by defects in retinal cilia, each with a similar, yet unique array of systemic symptoms and defining characteristics. A non-exhaustive list is provided in Figure 1.4,5
| Leber Congenital Amaurosis |
| Retinitis Pigmentosa |
| Usher Syndrome |
| Senior Løken Syndrome |
| Joubert Syndrome |
| Bardet-Biedl Syndrome |
| Alstrom Syndrome |
| Jeune Syndrome |
| Meckel Syndrome |
Table 1
A non-exhaustive list of various ciliopathies that lead to retinal disease.
Structure and function of primary cilia:
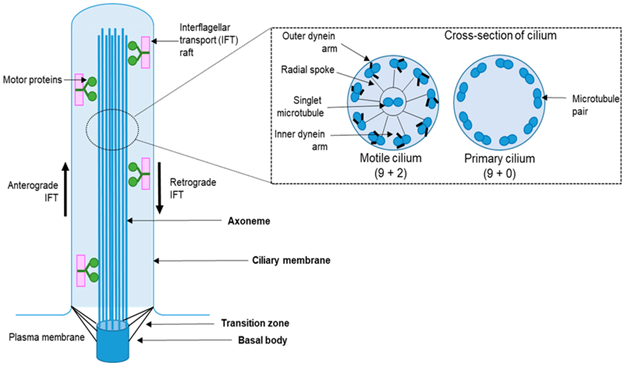
Cilia are characterized by a microtubule scaffolding that is essential to their overall structure and function. Primary cilia are arranged in a 9+0 configuration, which describes the manner in which the microtubules are organized radially in the cilium.6 It means there are 9 pairs of microtubules which run lengthwise from a centriole-like structure called the basal body to the distal end of the cilium. Motor proteins can perform both anterograde and retrograde transport in the cilium through a process called intraflagellar transport (IFT) by moving cargo attached to an IFT particle, as demonstrated in Figure 1.1
Figure 1
Diagram of the structure of the cilium. On the left a longitudinal section of the cilium is shown, with key structural components labeled. On the right is a cross-section of a motile cilium (left) and primary cilium (right), showing the microtubule structure and arrangement.
Cilia estructura.png by Maulin M. Patel ; Leonidas Tsiokas under license http://creativecommons.org/licenses/by/4.0/
Pathophysiology:
In the retina, primary cilia play a critical role in the health and function of retinal photoreceptor cells. They reside in the connecting cilium band of the retina and serve as a bridge between the inner and outer segments of the retinal photoreceptors.3,4 They transport proteins critical for phototransduction to the outer segment from the location of protein synthesis, the inner segment.3,4 They also serve an important role in the structural integrity of the retina.7 Therefore, mutations in genes that encode components of these vital structures can lead to degeneration of photoreceptors, and in turn cause significant vision loss.6
Treatment and Prognosis:
Historically, there has been no treatment to stop the progression of visual loss in inherited ciliopathies. Management was focused on maximizing a patient’s function by providing resources to live a fruitful life with greatly diminished visual acuity. However, given new technologies such as gene therapy, there is renewed hope that patients with these diseases can avoid progressive visual loss throughout life. The first ever gene therapy for inherited retinal diseases was approved in the United States in December 2017, when the Food and Drug Administration (FDA) approved the use of Luxturna (voretigene neparvovic-rzyl) for patients with retinal dystrophy due to bi-allelic RPE65 mutation.8 Gene therapies used to treat other inherited retinal diseases are under active research, discovery, and clinical trial, marking a hopeful future for patients worldwide.9
Summary of the Case:
Our patient had a genetic mutation in IFT-140, a gene critical for cilia health and function. Therefore, she has undergone progressive retinal degeneration and has a VA of approximately 20/400. Because primary cilia are present in many organs of the body, her disease is systemic, and is also causing nephropathy and musculoskeletal challenges. New developments in technology such as gene therapy have created novel treatments for inherited ciliopathies that will likely continue to expand in scope and efficacy.
References:
- Satir P, Christensen S. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377-400. doi: 10.1146/annurev.physiol.69.040705.141236. PMID: 17009929.
- Adams, M. The Primary Cilium: An Orphan Organelle Finds a Home. Nature Education 2010 3(9):54
- Bujakowska KM, Liu Q, Pierce EA. Photoreceptor Cilia and Retinal Ciliopathies. Cold Spring Harb Perspect Biol. 2017 Oct 3;9(10):a028274. doi: 10.1101/cshperspect.a028274. PMID: 28289063; PMCID: PMC5629997
- Chen HY, Welby E, Li T, Swaroop A. Retinal disease in ciliopathies: Recent advances with a focus on stem cell-based therapies. Transl Sci Rare Dis. 2019 Jul 4;4(1-2):97-115. doi: 10.3233/TRD-190038. PMID: 31763178; PMCID: PMC6839492.
- Fuster-García C, García-Bohórquez B, Rodríguez-Muñoz A, Aller E, Jaijo T, Millán J, García-García G. Usher Syndrome: Genetics of a Human Ciliopathy. Int J Mol Sci. 2021 Jun 23;22(13):6723. doi: 10.3390/ijms22136723. PMID: 34201633; PMCID: PMC8268283.
- Hoey D, Downs M, Jacobs C. The mechanics of the primary cilium: an intricate structure with complex function. J Biomech. 2012 Jan 3;45(1):17-26. doi: 10.1016/j.jbiomech.2011.08.008. Epub 2011 Sep 6. PMID: 21899847; PMCID: PMC3242821.
- Mercey O, Kostic C, Bertiaux E, Giroud A, Sadian Y, Gaboriau DCA, Morrison CG, Chang N, Arsenijevic Y, Guichard P, Hamel V. The connecting cilium inner scaffold provides a structural foundation that protects against retinal degeneration. PLoS Biol. 2022 Jun 16;20(6):e3001649. doi: 10.1371/journal.pbio.3001649. PMID: 35709082; PMCID: PMC9202906.
- Hsu, J. Voretigene neparvovec-rzyl (Luxturna™), EyeWiki 2024. Available at https://eyewiki.aao.org/Voretigene_neparvovec-rzyl_(Luxturna%E2%84%A2). Accessed August 1, 2024.
- Nuzbrokh Y, Ragi S, Tsang S. Gene therapy for inherited retinal diseases. Ann Transl Med. 2021 Aug;9(15):1278. doi: 10.21037/atm-20-4726. PMID: 34532415; PMCID: PMC8421966.
Faculty Approval by: Griffin Jardine, MD
Copyright statement: Tanner Nelson, ©2024. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/