Enhanced S-cone Syndrome: an NR2E3-Associated Retinal Dystrophy
Home / Retina and Vitreous / Hereditary and Choroidal Dystrophies
Title: Enhanced S-cone Syndrome: an NR2E3-Associated Retinal Dystrophy
Authors: Aniket Ramshekar, PhD, MSIV, University of Utah School of Medicine and Cecinio “Nikko” Ronquillo, MD, PhD
Date: 8/23/23
Keywords/Main Subjects: enhanced s-cone syndrome, NR2E3, NRL, nyctalopia, photoreceptors
Diagnosis: Enhanced S-cone Syndrome
Description of Case: A 13-year-old male presented with impaired nighttime vision (i.e., nyctalopia) and peripheral vision loss. Past ocular history is notable for glasses since he was 4 years old. Medical and developmental history were unremarkable. Family history was unremarkable.
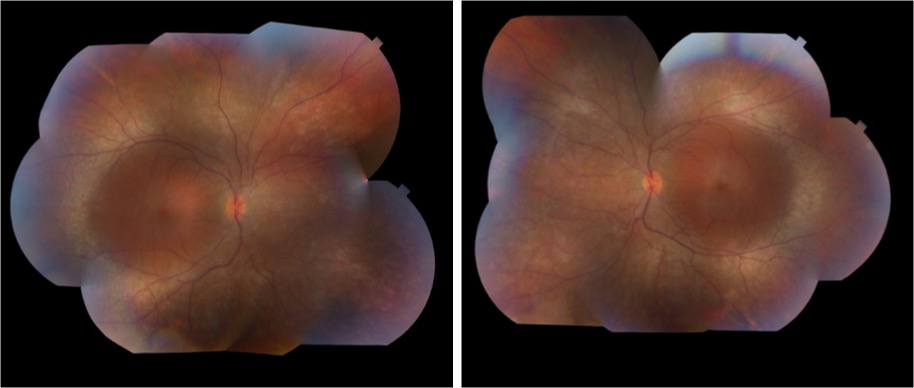
On exam, the patient had best corrected visual acuity (BCVA) of 20/30 OD and 20/40 OS, intraocular pressure (IOP) of 12 OD and 9 OS, and reduced peripheral vision to confrontation OU. Fundus exam was notable for loss of pigment, grayish appearance around the arcades and peripheral retina associated with nummular pigment changes along the superior arcade OS (Figure 1).
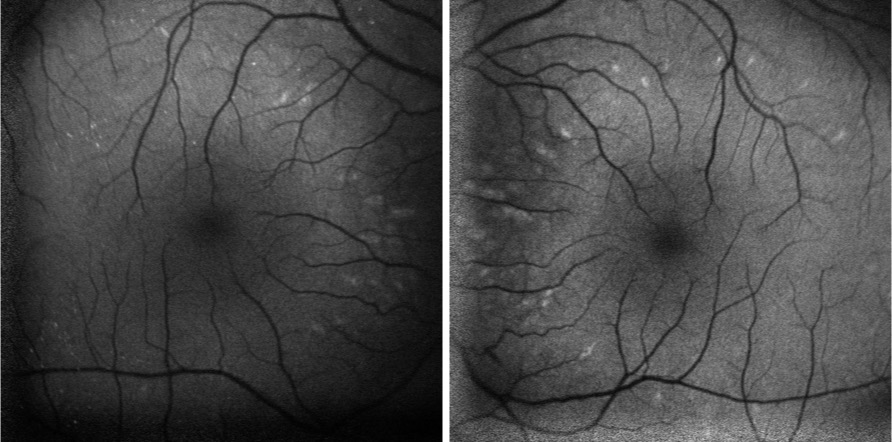
Fundus autofluorescence revealed hyper-autofluorescent spots surrounding the fovea OU (Figure 2).
Optical coherence tomography (OCT) demonstrated retained inner layers of the retina OU, cystic changes in the outer plexiform layer (OPL) OU, and loss of definition of the outer retinal layers OU (Figure 3).
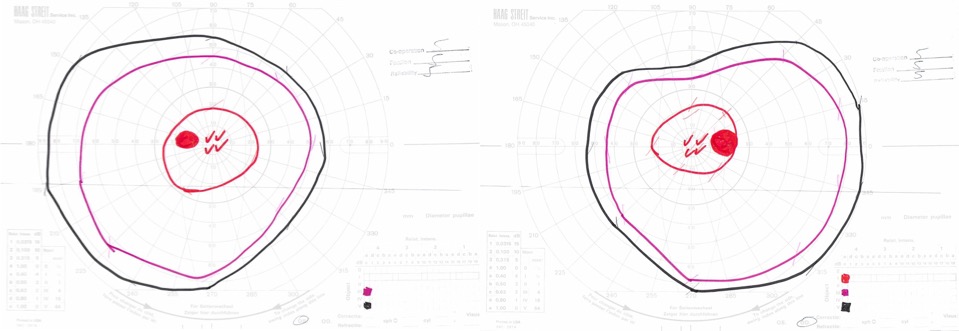
Goldmann visual field exam was full (Figure 4).
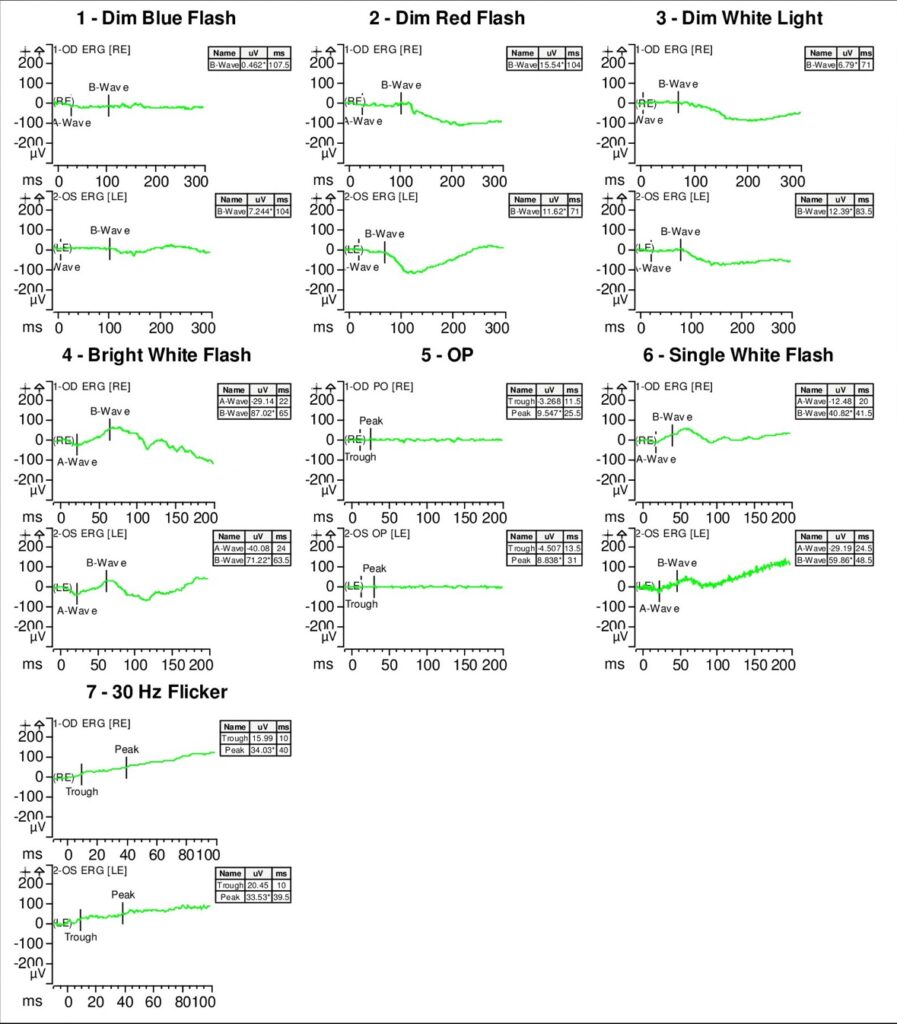
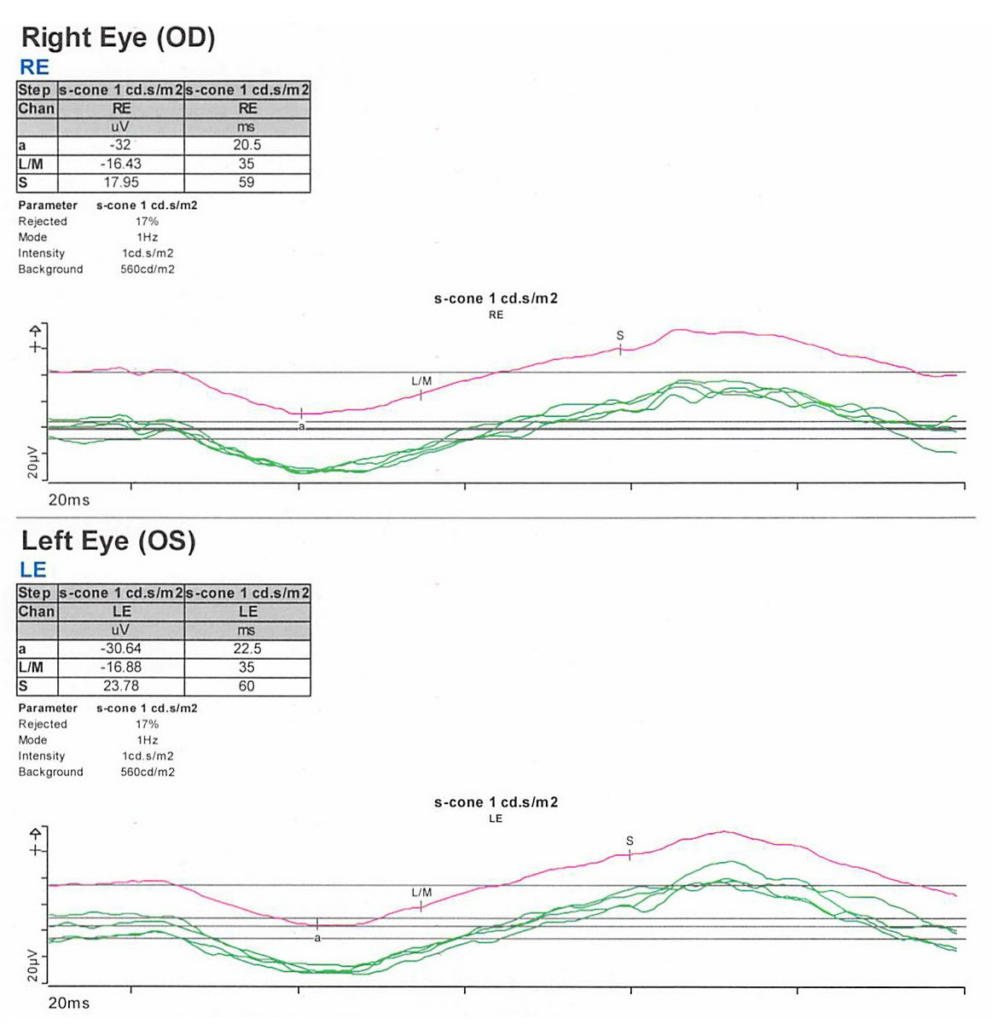
Full-field electroretinogram (ERG) showed diminished scotopic and photopic bright white flashes OU and 30 Hz flicker response that was delayed and diminished in amplitude OU (Figure 5).
Given the concern for inherited retinal dystrophy, the patient was referred for genetic testing that revealed a homozygous NR2E3 c.119-2A>C variant, which disrupts the nearby universal “AG” splice acceptor site and is likely pathogenic.
S-cone ERG, acquired in response to a blue wavelength on an orange wavelength background, demonstrated a greater than anticipated response OU (Figure 6).
Epidemiology: Enhanced S-cone syndrome (ESCS) is a rare inherited progressive retinal degeneration. The prevalence of ESCS is not well-established; however, studies have found gene mutations associated with ESCS worldwide.1-12
Genetics: ESCS is inherited in an autosomal recessive pattern. NR2E3 (Nuclear Receptor Subfamily 2, Group E, Member 3) mutations are primarily implicated in ESCS. There are over 30 pathogenic mutations in NR2E3 that have been associated with ESCS.13-15 In the United States, however, the NR2E3 c.119-2A>C variant is the most common.16
Some ESCS cases have also documented NRL (neural retina leucine zipper) mutations.17-20
Pathophysiology: NR2E3 protein is a retinal orphan nuclear receptor, a transcription factor expressed in the outer nuclear layer of the human retina.13 To gain mechanistic insights, NR2E3 mutations in mouse models have been shown to disrupt the development of rod photoreceptors and disrupt the differentiation of cone photoreceptors to L/M-cones leading to over-expansion of the S-cone population in the retina.21-23 NR2E3 expression is under the direct regulation of the transcription factor, NRL protein.24 Therefore, mutations in NRL lead to ESCS by affecting NR2E3 expression.25
Clinical presentation: The clinical presentation of ESCS is variable; however, listed below are some documented symptoms.26-28
- Night blindness (nyctalopia, due to reduced functioning rod photoreceptors)
- Increased sensitivity to blue light (due to the over-expansion of functioning S-cones)
- Reduced visual acuity
- Abnormal color vision, with a tendency to see colors as more vibrant or intense
Diagnosis: Given that the clinical presentation of ESCS is variable, the diagnostic testing might also demonstrate variable findings among patients. Below are some diagnostic findings associated with each clinical test:
- Fundus exam – Fundus exam might demonstrate torpedo-like lesions primarily located along the vascular arcades, subretinal fibrosis, nummular pigmentary changes along the vascular arcades, or intraretinal yellow dots.6,29
- ERG – full-field ERG might demonstrate a diminished rod response and similar waveform in response to both scotopic and photopic conditions. The 30 Hz flicker responses might demonstrate significantly delayed responses with attenuated amplitudes. With orange background, increased response to short wavelength (blue) might be elicited. The multifocal ERG (mfERG) might show preserved central responses, though can be delayed.6,30-35
- OCT – OCT might demonstrate cystoid macular edema (CME) or foveomacular schisis.31 There are some documented cases of subretinal fibrosis secondary to choroidal neovascularization that might also be detected by OCT.6,35-27 There was also a case of macular retinal vascularization of the posterior pole that was detected by OCT.38
- FA – In the peripheral retina, FA might demonstrate hypo-autofluorescence with patchy areas of advanced hypo-autofluorescence. In the macula, hyper-autofluorescent flecks have been documented.6 FA might also identify vascular changes (i.e., retinal or choroidal neovascularization) and complement OCT findings.
- Genetic testing can identify mutations in the NR2E3 or NRL genes and confirm the diagnosis
Differential diagnosis: Nyctalopia has a broad differential and includes conditions such as Vitamin A deficiency, cataracts, autoimmune retinopathy, glaucoma, and other inherited retinal dystrophies (i.e., congenital stationary night blindness, Oguchi’s disease, fundus albipunctatus). Therefore, a careful workup is required to narrow the differential to treat patients appropriately.
Some NR2E3 mutations are implicated in autosomal dominant retinitis pigmentosa, Goldmann-Favre syndrome, and clumped pigmentary retinal degeneration.39-41 Characterizing the underlying disease caused by the NR2E3 mutations might not improve medical management but would inform genetic counseling.
Management: There are currently no approved therapies to treat ESCS. However, CRISPR/Cas9 gene editing has been used preclinically to correct the homozygous NR2E3 c.119-2A>C splice site variant in induced pluripotent stem cells (iPSCs) from two patients with ESCS.42 This finding suggests that CRISPR/Cas9 gene editing might be a plausible treatment approach for ESCS in the future.
Current medical management includes treating foveomacular schisis and CME with carbonic anhydrase inhibitors, while choroidal neovascularization is treated with agents that inhibit vascular endothelial growth factor.43-49 Genetic testing and patient support programs should also be offered in all patient cases. Genetic penetrance and carrier frequency of the NR2E3 mutations are not well understood given the rarity of disease phenotypes. Therefore, genetic testing of the patient and family might help provide further information to improve genetic counseling.
Summary of the Case:
- ESCS is a rare inherited progressive retinal degenerative disease of the photoreceptors that can present as nyctalopia
- There is clinical and diagnostic variability among patients with ESCS, but mutations in the transcription factors, NR2E3 or NRL, are implicated in pathology
- Current treatment is limited to treating foveomacular schisis and CME with carbonic anhydrase inhibitors, and choroidal neovascularization with anti-VEGF agents
- Genetic testing should be offered to inform genetic counseling
- Studies exploring CRISPR/Cas9 gene editing show promise for future therapies
References
- Bandah, D., Merin, S., Ashhab, M., Banin, E. & Sharon, D. The spectrum of retinal diseases caused by NR2E3 mutations in Israeli and Palestinian patients. Arch Ophthalmol 127, 297-302, doi:10.1001/archophthalmol.2008.615 (2009).
- Gao, F. J. et al. Genetic and Clinical Findings in a Large Cohort of Chinese Patients with Suspected Retinitis Pigmentosa. Ophthalmology 126, 1549-1556, doi:10.1016/j.ophtha.2019.04.038 (2019).
- Alsalamah, A. K., Khan, A. O., Bakar, A. A., Schatz, P. & Nowilaty, S. R. Recognizable Patterns of Submacular Fibrosis in Enhanced S-Cone Syndrome. Ophthalmol Retina 5, 918-927, doi:10.1016/j.oret.2021.03.014 (2021).
- Habibi, I. et al. Different Phenotypes in Pseudodominant Inherited Retinal Dystrophies. Front Cell Dev Biol 9, 625560, doi:10.3389/fcell.2021.625560 (2021).
- Al-Khuzaei, S. et al. Novel Pathogenic Sequence Variants in NR2E3 and Clinical Findings in Three Patients. Genes (Basel) 11, doi:10.3390/genes11111288 (2020).
- de Carvalho, E. R. et al. Enhanced S-Cone Syndrome: Spectrum of Clinical, Imaging, Electrophysiologic, and Genetic Findings in a Retrospective Case Series of 56 Patients. Ophthalmol Retina 5, 195-214, doi:10.1016/j.oret.2020.07.008 (2021).
- Hebbar, P. et al. Genetic risk variants for metabolic traits in Arab populations. Sci Rep 7, 40988, doi:10.1038/srep40988 (2017).
- Van Cauwenbergh, C. et al. Mutations in Splicing Factor Genes Are a Major Cause of Autosomal Dominant Retinitis Pigmentosa in Belgian Families. PLoS One 12, e0170038, doi:10.1371/journal.pone.0170038 (2017).
- Kuniyoshi, K. et al. New truncation mutation of the NR2E3 gene in a Japanese patient with enhanced S-cone syndrome. Jpn J Ophthalmol 60, 476-485, doi:10.1007/s10384-016-0470-0 (2016).
- Kannabiran, C., Singh, H., Sahini, N., Jalali, S. & Mohan, G. Mutations in TULP1, NR2E3, and MFRP genes in Indian families with autosomal recessive retinitis pigmentosa. Mol Vis 18, 1165-1174 (2012).
- Udar, N., Small, K., Chalukya, M., Silva-Garcia, R. & Marmor, M. Developmental or degenerative–NR2E3 gene mutations in two patients with enhanced S cone syndrome. Mol Vis 17, 519-525 (2011).
- Naik, A. et al. Enhanced S-cone syndrome: Clinical spectrum in Indian population. Indian J Ophthalmol 67, 523-529, doi:10.4103/ijo.IJO_1480_18 (2019).
- Haider, N. B. et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet 24, 127-131, doi:10.1038/72777 (2000).
- Schorderet, D. F. & Escher, P. NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP). Hum Mutat 30, 1475-1485, doi:10.1002/humu.21096 (2009).
- Escher, P. et al. Mutations in NR2E3 can cause dominant or recessive retinal degenerations in the same family. Hum Mutat 30, 342-351, doi:10.1002/humu.20858 (2009).
- Stone, E. M. et al. Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology 124, 1314-1331, doi:10.1016/j.ophtha.2017.04.008 (2017).
- Iarossi, G. et al. A Novel Autosomal Recessive Variant of the NRL Gene Causing Enhanced S-Cone Syndrome: A Morpho-Functional Analysis of Two Unrelated Pediatric Patients. Diagnostics (Basel) 12, doi:10.3390/diagnostics12092183 (2022).
- Newman, H. et al. Homozygosity for a Recessive Loss-of-Function Mutation of the NRL Gene Is Associated With a Variant of Enhanced S-Cone Syndrome. Invest Ophthalmol Vis Sci 57, 5361-5371, doi:10.1167/iovs.16-19505 (2016).
- Nishiguchi, K. M. et al. Recessive NRL mutations in patients with clumped pigmentary retinal degeneration and relative preservation of blue cone function. Proc Natl Acad Sci U S A 101, 17819-17824, doi:10.1073/pnas.0408183101 (2004).
- Littink, K. W. et al. Autosomal Recessive NRL Mutations in Patients with Enhanced S-Cone Syndrome. Genes (Basel) 9, doi:10.3390/genes9020068 (2018).
- Haider, N. B., Naggert, J. K. & Nishina, P. M. Excess cone cell proliferation due to lack of a functional NR2E3 causes retinal dysplasia and degeneration in rd7/rd7 mice. Hum Mol Genet 10, 1619-1626, doi:10.1093/hmg/10.16.1619 (2001).
- Haider, N. B. et al. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp Eye Res 89, 365-372, doi:10.1016/j.exer.2009.04.006 (2009).
- Haider, N. B. et al. The transcription factor Nr2e3 functions in retinal progenitors to suppress cone cell generation. Vis Neurosci 23, 917-929, doi:10.1017/S095252380623027X (2006).
- Oh, E. C. et al. Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors. Brain Res 1236, 16-29, doi:10.1016/j.brainres.2008.01.028 (2008).
- Mears, A. J. et al. Nrl is required for rod photoreceptor development. Nat Genet 29, 447-452, doi:10.1038/ng774 (2001).
- Khan, A. O., Aldahmesh, M. & Meyer, B. The enhanced S-cone syndrome in children. BMJ Case Rep 2009, doi:10.1136/bcr.10.2008.1163 (2009).
- Fishman, G. A., Jampol, L. M. & Goldberg, M. F. Diagnostic features of the Favre-Goldmann syndrome. Br J Ophthalmol 60, 345-353, doi:10.1136/bjo.60.5.345 (1976).
- Garafalo, A. V. et al. Cone Vision Changes in the Enhanced S-Cone Syndrome Caused by NR2E3 Gene Mutations. Invest Ophthalmol Vis Sci 59, 3209-3219, doi:10.1167/iovs.18-24518 (2018).
- Yzer, S. et al. Expanded clinical spectrum of enhanced S-cone syndrome. JAMA Ophthalmol 131, 1324-1330, doi:10.1001/jamaophthalmol.2013.4349 (2013).
- Tsang, S. H. & Sharma, T. Enhanced S-Cone Syndrome (Goldmann-Favre Syndrome). Adv Exp Med Biol 1085, 153-156, doi:10.1007/978-3-319-95046-4_28 (2018).
- Vincent, A., Robson, A. G. & Holder, G. E. Pathognomonic (diagnostic) ERGs. A review and update. Retina 33, 5-12, doi:10.1097/IAE.0b013e31827e2306 (2013).
- Hood, D. C., Cideciyan, A. V., Roman, A. J. & Jacobson, S. G. Enhanced S cone syndrome: evidence for an abnormally large number of S cones. Vision Res 35, 1473-1481, doi:10.1016/0042-6989(95)98727-q (1995).
- Jacobson, S. G., Marmor, M. F., Kemp, C. M. & Knighton, R. W. SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci 31, 827-838 (1990).
- Marmor, M. F., Jacobson, S. G., Foerster, M. H., Kellner, U. & Weleber, R. G. Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am J Ophthalmol 110, 124-134, doi:10.1016/s0002-9394(14)76980-6 (1990).
- Audo, I. et al. Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci 49, 2082-2093, doi:10.1167/iovs.05-1629 (2008).
- Nakamura, M. et al. Enhanced S-cone syndrome with subfoveal neovascularization. Am J Ophthalmol 133, 575-577, doi:10.1016/s0002-9394(01)01428-3 (2002).
- Zerbib, J. et al. Retinochoroidal Anastomosis Associated with Enhanced S-Cone Syndrome. Retin Cases Brief Rep 13, 295-299, doi:10.1097/ICB.0000000000000594 (2019).
- Bazvand, F., Khojasteh, H. & Zarei, M. Novel findings in enhanced S-cone syndrome: a case with macular retinal neovascularization and severe retinal vasculitis. Doc Ophthalmol 139, 221-226, doi:10.1007/s10633-019-09706-6 (2019).
- Sharon, D., Sandberg, M. A., Caruso, R. C., Berson, E. L. & Dryja, T. P. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol 121, 1316-1323, doi:10.1001/archopht.121.9.1316 (2003).
- Chavala, S. H. et al. An Arg311Gln NR2E3 mutation in a family with classic Goldmann-Favre syndrome. Br J Ophthalmol 89, 1065-1066, doi:10.1136/bjo.2005.068130 (2005).
- Gire, A. I. et al. The Gly56Arg mutation in NR2E3 accounts for 1-2% of autosomal dominant retinitis pigmentosa. Mol Vis 13, 1970-1975 (2007).
- Bohrer, L. R. et al. Correction of NR2E3 Associated Enhanced S-cone Syndrome Patient-specific iPSCs using CRISPR-Cas9. Genes (Basel) 10, doi:10.3390/genes10040278 (2019).
- Iannaccone, A., Fung, K. H., Eyestone, M. E. & Stone, E. M. Treatment of adult-onset acute macular retinoschisis in enhanced s-cone syndrome with oral acetazolamide. Am J Ophthalmol 147, 307-312 e302, doi:10.1016/j.ajo.2008.08.003 (2009).
- Chatzistergiou, V. et al. Optical Coherence Tomography Analysis of Cystoid Macular Edema in Retinal Dystrophy Treated with Oral Acetazolamide: Two Cases. Klin Monbl Augenheilkd 237, 484-486, doi:10.1055/a-1068-2762 (2020).
- Genead, M. A., Fishman, G. A. & McAnany, J. J. Efficacy of topical dorzolamide for treatment of cystic macular lesions in a patient with enhanced S-cone syndrome. Doc Ophthalmol 121, 231-240, doi:10.1007/s10633-010-9247-9 (2010).
- Hajali, M. & Fishman, G. A. Dorzolamide use in the management of macular cysts in a patient with enhanced s-cone syndrome. Retin Cases Brief Rep 3, 121-124, doi:10.1097/ICB.0b013e31818faa21 (2009).
- Bechet, L. et al. Management of a case of Enhanced S-cone syndrome with massive foveoschisis treated with pars plana vitrectomy with silicone oil tamponade. Ophthalmic Genet 42, 615-618, doi:10.1080/13816810.2021.1925927 (2021).
- Broadhead, G. K., Grigg, J. R., McCluskey, P., Korsakova, M. & Chang, A. A. Bevacizumab for choroidal neovascularisation in enhanced S-cone syndrome. Doc Ophthalmol 133, 139-143, doi:10.1007/s10633-016-9555-9 (2016).
- Bertoli, F., Pignatto, S., Rizzetto, F. & Lanzetta, P. A 5-Year-Old Case of Choroidal Neovascularization in Enhanced S-Cone Syndrome Treated with Ranibizumab. Case Rep Ophthalmol 9, 510-515, doi:10.1159/000495743 (2018).
Faculty Approval by: Paul Bernstein, MD, PhD
Copyright: Copyright Ramshekar and Ronquillo, ©2023. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
Identifier: Moran_CORE_126913