Essentials of Biometry (Part 2): How are biometric measurements obtained?
Home / Lens and Cataract / Evaluation and Management of Cataracts in Adults
Title: Essentials of Biometry (Part 2): How are biometric measurements obtained?
Authors: Courtney Goodman, MSIV at University of Miami Miller School of Medicine, Mark Mifflin, MD
Date: 11/14/2022
Keywords/Main Subjects: biometry; preoperative measurements; cataract surgery; topography
Review:
In this next article, we will review the instruments used to measure biometry today as well as historically. We will also discuss the pros and cons of each instrument.
Today, the most common and accurate method for obtaining biometric measurements in developed countries is laser interferometry (Figure 1).1 These devices center a laser onto the macula to obtain rapid, non-invasive, and automated (meaning, human control is minimized) measurements. Older generations used partial coherence interferometry (PCI) or optical low-coherence reflectometry (OLCR), while newer generations employ swept-source optical coherence tomography (SS-OCT) that additionally provides an image to ensure proper fixation.2,3 Newer models also have a built-in keratometer which calculates K and measures astigmatism at multiple reference points. These evolving innovations have unprecedentedly allowed the two most important measurements, axial length (AL) and central corneal power (K), to be obtained using a single device, among other measurements. Conveniently, intraocular lens (IOL) powers are then automatically calculated using the various available formulas.

Figure 1: Laser Interferometer. Laser interferometry is the most common method for obtaining biometric measurements today. The Lenstar laser interferometer is depicted, but it should be noted that various brands exist that work on the same principles. Photographer: Courtney Goodman.
While laser interferometry has clear advantages, the devices are relatively expensive. Therefore, older methods for obtaining AL and K are still widely used, especially in developing countries. Apart from lack of access, alternative methods are also sometimes necessary when a patient has a dense cataract through which a laser cannot pass through, or has problems with ocular fixation. For these reasons, it is important to be familiar with conventional biometry methods, such as ultrasonic methods using the A-scan.
A-scan ultrasound biometry used to be the gold standard in measuring AL, and still maintains good accuracy measuring with sound waves rather than laser light.4 Since sound has a longer wavelength, ultrasonic methods yield lower-resolution measurements compared to laser interferometry. Another difference is that A-scan ultrasound biometry measures to the internal limiting membrane rather than the photoreceptor layer. Therefore, a standard retinal length is usually added to generate the final AL, a step which is theoretically less accurate.5 There are two techniques of A-scan: 1) contact applanation and 2) immersion. Contact A-scan involves lightly touching a probe onto the corneal surface, which has a risk of compressing the cornea and shortening the AL. Immersion A-scan reduces this risk by submerging the probe into a saline bath within a scleral shell rather than directly contacting the cornea (Figure 2). As can be expected, A-scan techniques are more prone to human error. With a skilled user, however, immersion A-scan ultrasound can have biometric accuracy on-par with laser interferometry.6

Figure 2: Immersion A-scan ultrasound. The patient is positioned supine, and a scleral shell filled with saline is placed onto the cornea. The A-scan probe is submerged into the saline bath and measures along the axial plane, which are displayed onto a graph. Created with Biorender.
While ultrasonic biometry could obtain measurements along the axial plane, separate methods were necessary to evaluate the corneal surface. Placido disc keratoscopy was the first advancement in corneal topography, invented in 1880. Black and white concentric rings are projected onto the corneal surface, allowing for qualitative assessment of the anterior curvature (Figure 3). Today, handheld Placido disc keratoscopes are still used to quickly assess for astigmatism in the clinic, such as for planning suture removals of an eye which underwent penetrating keratoplasty. K was not able to be calculated, however, until quantitative techniques emerged, including manual keratometry, and the computerized analyses scanning-slit tomography, Scheimpflug tomography, and Placido disc-based topography.
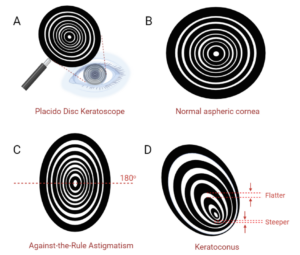
Figure 3 Placido disc keratoscopy. Placido disc keratoscopy is a qualitative method for assessing corneal topography. A) A handheld Placido disc is approached to the eye until the black-and-white concentric rings reflect off the corneal surface. B) A normal aspheric cornea will have reflections that appear similar to the Placido disc itself, with equal spacing between the mires (the spaces between the rings). More widely-spaced mires indicate a flatter surface, while closely-spaced mires indicate a steeper shape. C) Against-the-rule astigmatism has a steeper axis at 180o, identified by the closer-spaced mires along that axis. D) Keratoconus presents variably, and here has a non-centric steepening and irregular mires. Created with Biorender.
Although the manual keratometer was invented in 1851, it was not used for cataract surgery until the mid-20th century when K was incorporated into IOL power formulas.7 The keratometer was the first device that measured the radius of the anterior corneal curvature to calculate K. The radius is derived by relating the true height of an object and the height of the image of the object projected by the central optical zone of the cornea (i.e., first Purkinje image) in a formula. Then, K can be calculated using radius and the refractive indices of the cornea and air (Figure 4). K calculations using this method are less accurate as posterior corneal curvature is not accounted for, and the calculations cannot adjust for an abnormal corneal shape.
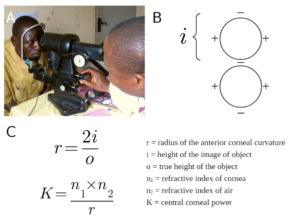
Figure 4 Manual Keratometry. Manual keratometry was the first method developed for measuring the radius of the anterior corneal curvature and calculating K. A) An example of a manual keratometer and B) the mires within the device are depicted. The handles are rotated until the doubled images seen through the scope contact, with the displacement being equal to the height of the object image. C) Two equations are then used to manually calculate the radius and K. Created with Biorender. Reference for Figure 4A: Mike Blyth, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons.
Corneal-scanning technologies emerged with the evolution of modern computers. Among the first developed was Placido disc-based topography, a technology to evaluate the anterior corneal surface (Figure 5). These devices measure the reflections of a Placido disc to provide colored anterior curvature maps, a Placido disc image, and corneal biometric calculations. Placido disc-based topographers have undergone considerable modernization over the years, with some devices additionally providing maps and measurements of the optical path difference (OPD), which detects corneal surface aberrations or globe distortions causing refractive error. While Placido disc-based topography does not evaluate the posterior cornea, it is still commonly used today to obtain quick anterior surface topographic maps and accurate measurements of K and astigmatism.
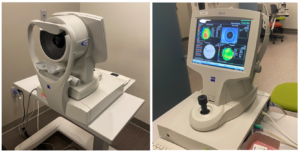
Figure 5 Placido disc-based topography. Placido disc-based topography generates a topographic map of the anterior corneal surface. The Zeiss Atlas topographer is depicted, which is one brand of many that are made by different manufacturers. Photographer: Courtney Goodman.
Entering the market in the late 1990s, scanning-slit topography was the first method that could map both anterior and posterior corneal surfaces and determine corneal thickness. The device projected 40 slit beams of light onto the cornea and a camera captured their reflection, from which colored corneal topography maps were generated.
Scheimpflug-based imaging has widely replaced scanning-slit technology, and provides similar data but through a different, more accurate method using rotary cameras (Figure 6). Scheimpflug imaging generates axial, tangential, elevation, refractive, pachymetry, and difference maps along with a 3D model of the cornea. Today, these maps are often used in routine cataract surgery evaluation to assess for astigmatism, and provide an alternate calculation of K in addition to laser interferometry. In some eyes, K derived from Scheimflug imaging is preferential, such as in keratoconus where it has been shown to minimize hyperopic surprise.8 Scheimpflug imaging is also critical for surgical planning of Toric lens implantation.9
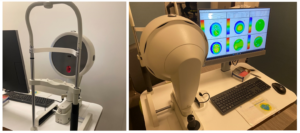
Figure 6: Scheimpflug-based imaging. In Scheimpflug-based imaging, topographic maps are generated using a rotary camera. The Oculus Pentacam is depicted as an example. Additional brands are made by different manufacturers. Photographer: Courtney Goodman.
The instruments used to measure biometry have undergone considerable evolution and increased in accuracy over the years, and new devices are continuously being developed. While most cataract surgery practices today are equipped with a laser interferometer, Scheimpflug device, and Placido disc-based topographer, it is nonetheless important to understand alternate methods, especially when conducting international work abroad.
References:
1. Kane JX, Chang DF. Intraocular Lens Power Formulas, Biometry, and Intraoperative Aberrometry: A Review. Ophthalmology. Nov 2021;128(11):e94-e114.
2. Guimaraes de Souza R, Montes de Oca I, Esquenazi I, Al-Mohtaseb Z, Weikert MP. Updates in Biometry. Int Ophthalmol Clin. Summer 2017;57(3):115-124.
3. Akman A, Asena L, Gungor SG. Evaluation and comparison of the new swept source OCT-based IOLMaster 700 with the IOLMaster 500. Br J Ophthalmol. Sep 2016;100(9):1201-1205.
4. Pereira A, Popovic M, Lloyd JC, El-Defrawy S, Schlenker MB. Preoperative measurements for cataract surgery: a comparison of ultrasound and optical biometric devices. Int Ophthalmol. Apr 2021;41(4):1521-1530.
5. Nakhli FR. Comparison of optical biometry and applanation ultrasound measurements of the axial length of the eye. Saudi J Ophthalmol. Oct 2014;28(4):287-291.
6. Naicker P, Sundralingam S, Peyman M, et al. Refractive outcomes comparison between the Lenstar LS 900(R) optical biometry and immersion A-scan ultrasound. Int Ophthalmol. Aug 2015;35(4):459-466.
7. Gutmark R, Guyton DL. Origins of the keratometer and its evolving role in ophthalmology. Surv Ophthalmol. Sep-Oct 2010;55(5):481-497.
8. Wang KM, Jun AS, Ladas JG, Siddiqui AA, Woreta F, Srikumaran D. Accuracy of Intraocular Lens Formulas in Eyes With Keratoconus. Am J Ophthalmol. Apr 2020;212:26-33.
9. Haddad JS, Barnwell E, Rocha KM, Ambrosio R, Jr., Waring Iv GO. Comparison of Biometry Measurements Using Standard Partial Coherence Interferometry versus New Scheimpflug Tomography with Integrated Axial Length Capability. Clin Ophthalmol. 2020;14:353-358.
Identifier: Moran_CORE_126595
Faculty Approval by: Dr. Mark Mifflin
Copyright statement: Copyright Courtney Goodman, ©2022. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/



