How to Assess Whether a Globe is Ruptured
Home / Basic Ophthalmology Review / Trauma
Title: How to Assess Whether a Globe is Ruptured
Author: Johnny Lippincott, 4th Year Medical Student, University of Mississippi Medical Center
Location: Medical Student Education Outline > II. Anatomical Approach to Eye Disease > Trauma > 2. How to assess whether a globe is ruptured
Text:
Introduction: An open globe or ruptured globe is an eyeball with a full-thickness defect in part of the eye’s wall, and is a true ophthalmic emergency. “Full-thickness” means all layers of the eye are disrupted. An open globe therefore allows communication between the eye’s interior and the environment. The danger of this communication is two-way: the eye’s internal contents can extrude, and environmental factors (e.g., pathogens) may enter.
If there is any concern that a patient may have an open globe, the top priority is avoiding further damage to the eye. This means special precautions must be taken immediately to assess whether this is the case. Until the integrity of the globe is confidently established, do not place external pressure of any sort on the eye of concern. Doing so can squeeze ocular contents through the defect and possibly cause permanent loss of vision or loss of the eye itself. Do not: patch the eye, measure intraocular pressure (e.g., with a Tonopen®), or allow the patient to rub the eye.
History
Patients may present with an open globe after various forms of trauma, from work- and sports-related injuries to falls and motor vehicle accidents. Particularly important history includes:
- Elicit possible mechanisms: was there a penetrating/perforating injury (e.g., BB gun shot), metal on metal contact without safety glasses or blunt trauma (e.g., racquetball)?
- Determine if there is potentially a foreign body based on the mechanism.
- Ask whether the patient has any prior history of eye surgery or trauma.
- Determine tetanus immunization status.
- Ask when the patient last ate or drank and instruct the patient to stop eating or drinking given they may likely need urgent surgery.
Signs of an Open Globe
A simple penlight is useful in grossly assessing the integrity of the globe.

Figure 1: Prolapsed iris through a corneal laceration with a very irregular pupil visible retina through the pupil. The conjunctiva is boggy and the entire globe appears deflated or slightly collapsed or simply “not round.”
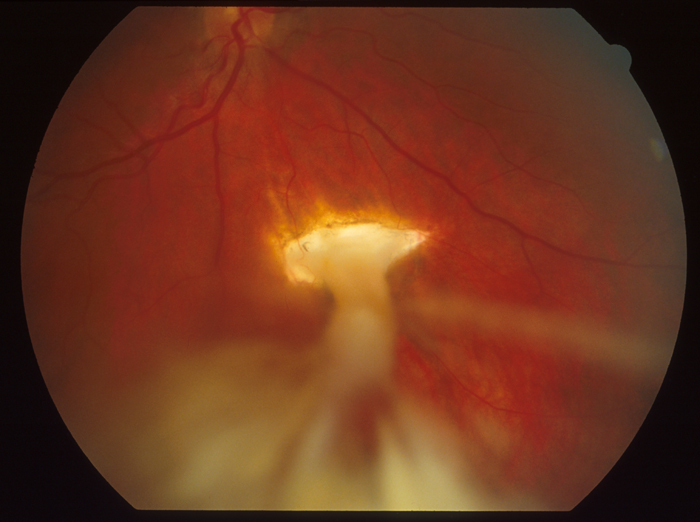
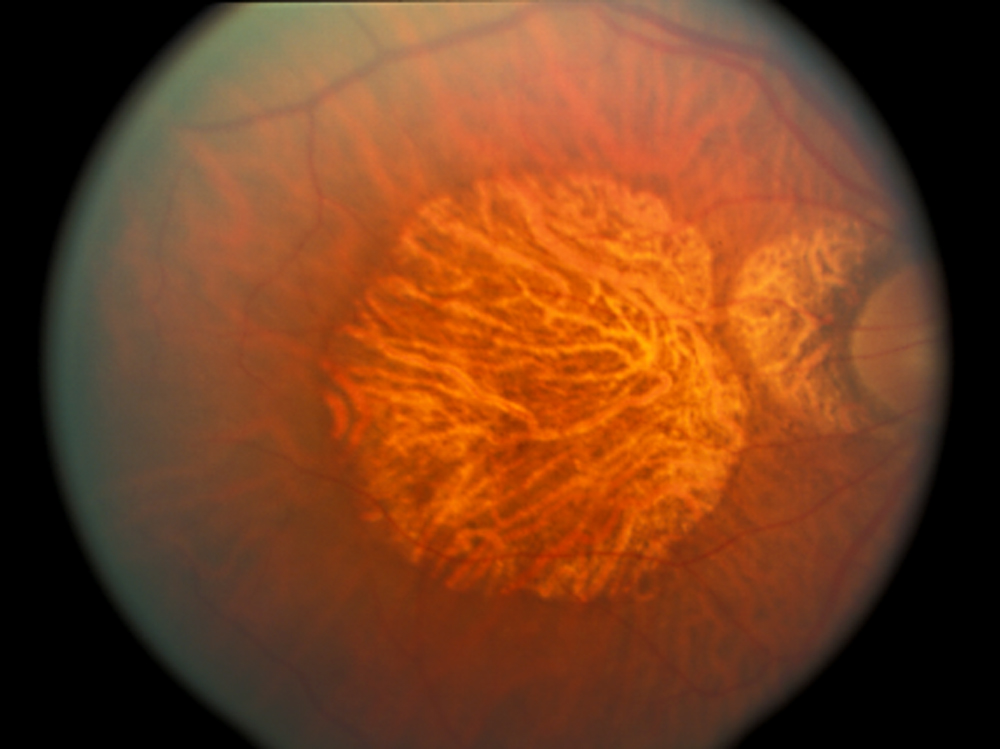
Image 2: Fundus photo of an inferior choroidal rupture versus scleral rupture with a vitreous tracking to the wound.
Pathognomonic signs:
- Full-thickness laceration(s) of the cornea or sclera
- Prolapse of intraocular contents (e.g., uveal tissue like the iris or ciliary body)
- Positive Seidel test (especially useful for known defect of unknown depth)
Three necessary tools for this test are 1) anesthetic eye drops (e.g., proparacaine HCl 0.5%), 2) fluorescein strip, and 3) cobalt blue light source with slit lamp/magnification. After numbing the eye with drops, gently paint dye across the wound. Looking under magnified cobalt blue light, either gross leakage from the defect or paling of the fluorescein dye wound indicate an active leak (indicating dilution of dye with intraocular fluid).
Suggestive signs:
- Severe 360º subconjunctival hemorrhage and/or chemosis (conjunctival edema)
- Flat, shallow or “deflated” looking anterior chamber compared to the fellow eye (always examine the other eye to rule out injury and for comparison)
- Teardrop pupil – an irregularly formed pupil (not round) often forms a point that clues you to where the rupture is it points in the direction of the prolapsed uvea.
- New or asymmetric cataract; dislocated lens material
- Motility defect in the affected eye
- CT scan (order CT scan of the “orbits” for thinner cuts through the globes and orbit) showing a globe that is not formed or round in comparison to the other eye
Treatment & Plan
Salient orders in the event of a suspicious or confirmed open globe include:
- Consult an ophthalmologist
- Shield the eye at all times except examination with something that vaults over the eye preventing any contact to the eye
- Make the patient 1) NPO (nil per os; nothing by mouth) to prepare for possible surgery, and 2) on bedrest with explicit instructions to avoid bending over, straining, or coughing
- Order broad spectrum antibiotics to prevent endophthalmitis and tetanus toxoid if the patient is overdue or has unclear immunization status
- Provide antiemetics and pain medication as needed
- CT scan of the brain and orbits are often indicated to rule out intraocular foreign bodies
References:
- Castellarin, A. A., & Pieramici, D. J. (2006). Open globe management. Comprehensive ophthalmology update, 8(3), 111-124.
- Ehlers, J. P., & Shah, C. P. (Eds.). (2008). The Wills eye manual: office and emergency room diagnosis and treatment of eye disease. Lippincott Williams & Wilkins.
- Harlan Jr, J. B., & Pieramici, D. J. (2002). Evaluation of patients with ocular trauma. Ophthalmology Clinics of North America, 15(2), 153-161.
- Seidel Test. Retrieved August 21, 2017, from http://eyewiki.org/Seidel_Test
Wet versus Dry Macular Degenerative Changes
Home / Basic Ophthalmology Review / Retina
Title: Wet versus Dry Macular Degenerative Changes
Author: Nina Boal, MSIV, Thomas Jefferson University
Photographer: James Gilman, CRA, FOPS
Location: Medical Student Education Outline > II. Anatomical Approach to Eye Disease > RETINA> 2. Wet versus Dry Macular degenerative changes
Overview
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in industrialized countries among people 50 years or older [1]. It is a degenerative disease of the macula (central portion of the retina), that results in central vision loss. Clinically, it is divided into dry (atrophic) or wet (exudative or neovascular). The risk of progressing from dry AMD to wet AMD is estimated at 1 to 4.7 percent in one year and 13 to 18 percent at three years [2].
Dry age-related Macular Degeneration
Macular changes in dry AMD are characterized by subretinal drusen deposits, atrophy of the retinal pigment epithelium (RPE), pigment epithelial detachments, and subretinal pigment epithelial clumping. There is an absence of neovascularization [3]. Dry AMD affects 85 to 90 percent of everyone with AMD [4].
- Drusen are deposits of extracellular material that appear as bright yellow spots on ophthalmoscopy, seen in figure 1. The risk for progression to wet AMD increases with increasing number and size of drusen and the presence of RPE pigmentary abnormalities. A few, small drusen is typical in people over 50 years old and are considered a normal part of aging [4].
- Retinal pigment epithelium (RPE) atrophy appears appear as clumps of hyperpigmentation or depigmented atrophic areas on ophthalmoscopy. Areas of loss of tissue and thinning can be focal or more widely dispersed on the macula, called geographic atrophy seen in figure 2.
Wet age-related Macular Degeneration
In wet (or neovascular) AMD, abnormal vessels grow into the subretinal space from the choroidal circulation. These vessels can leak and lead to subretinal hemorrhage, seen in figure 3, and subretinal fluid collections, indicating choroidal neovascularization. The goal is to recognize these new vessels before they bleed and cause a hemorrhagic detachment of the retinal pigment epithelium. Wet AMD is less common than dry AMD, affecting only 10 to 15 percent of people with AMD. However, it accounts for more than 80% of patients with severe visual loss or legal blindness [4].

Figure 3B. Fluorescein dye retinal angiography of patient with wet AMD, area of leakage corresponds to hemorrhage in figure 3A
Presentation
The main symptom of AMD is loss of central vision, but initially AMD may be asymptomatic. Patients with dry AMD describe a gradual loss of vision in the center of their visual field. Patients with wet AMD may describe a more acute visual distortion or loss of central vision as fluid or blood accumulates under the retina.
Work-up
- Visual Acuity
- Dilated eye examination
- Amsler grid
- A useful tool, seen in figure 4, to evaluate the functioning of the macula.
- The patient focuses one eye at a time on the center dot of the grid from 1 foot away, and then notes irregularities in the lines.
- This test specifically tests for metamophopsia (distortion of straight lines), an early change in wet AMD [3].
- Fluorescein dye retinal angiography
- Can be helpful to identify neovascularization in wet AMD
- Fluorescein dye is injected intravenously, and a sequence of photographs are taken. Newly formed choroidal neovascular vessels will leak fluorescein as seen in figure 3B.
- Optical coherence tomography (OCT)
- Produces cross sectional images of the retina and can be used to identify drusen, retinal edema, and subretinal fluid.
Treatment of Dry AMD
There is no proven effective treatment for dry AMD, however these patients may eventually develop wet AMD [3]. To prevent this progression, patients can be advised to stop smoking and take a combination of vitamins and minerals that make up the AREDS formula (Age-Related Eye Disease Study). A combination of antioxidant vitamins plus zinc was shown to protect the eye from further damage from AMD in patients who had more extensive dry and wet AMD [5,6].
Treatment of Wet AMD
In addition to the use of antioxidants and zinc, treatment of wet AMD attempts to stop and prevent neovascularization through:
- VEGF inhibitors
- Central to treatment of wet AMD, anti-VEGF therapies such as bevacizumab, ranibizumab, and aflibercept are injected into the vitreous monthly or bimonthly
- VEGF plays an important role in neovascularization. By inhibiting VEGF, the progression of wet AMD is stopped and vision loss can be stabilized or improved [7].
- Photodynamic therapy (PDT)
- Typically used if anti-VEGF therapy is not effective
- Involves intravenous injection of the photosensitization dye verteporfin before treating the eye with a photo-activating laser. The role of this therapy has decreased with the increasing use of anti-VEGF therapy [3].
- Thermal laser photocoagulation
- Use is limited to smaller lesions outside of the central macula due to the risk of scotoma and vision loss [3].
Summary Table
| Dry AMD | Wet AMD |
| 85 to 90% of patients with AMD | 10 to 15% of patients with AMD |
| Absence of neovascularization | Choroidal neovascularization– subretinal hemorrhage and subretinal fluid collections |
| Drusen, RPE atrophy, pigment epithelial detachments, subretinal pigment epithelial clumping | Drusen, RPE atrophy, pigment epithelial detachments, subretinal pigment epithelial clumping |
| Slow progression | Rapid loss of central vision over weeks to months |
| Mild to Severe central vision loss | More severe vision loss or legal blindness |
| Treatment:
– Monitor progression to wet AMD with Amsler grid – Smoking cessation – AREDS antioxidants and zinc supplements (more effective for extensive dry AMD) |
Treatment:
– Smoking cessation – AREDS antioxidants and zinc supplements – Anti-VEGF therapy – If anti-VEGF therapy does not work consider PDT or thermal laser photocoagulation |
Faculty Reviewer: Griffin Jardine, MD
References:
- Hyman L. Epidemiology of eye disease in the elderly. Eye (Lond) 1987; 1 ( Pt 2):330.
- Bressler NM. Age-related macular degeneration is the leading cause of blindness… JAMA 2004; 291:1900.
- Lietman MW. Manual for eye examination and diagnosis. 9th Hoboken, NJ: John Wiley & Sons Inc.; 2017.
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med 2008; 358:2606.
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001; 119:1417.
- Tan JS, Mitchell P, Kifley A, et al. Smoking and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol 2007; 125:1089.
- Solomon SD, Lindsley K, Vedula SS, et al. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2014.
Identifier: Moran_CORE_24645
The Motility Exam
Home / Basic Ophthalmology Review / Extraocular Motility
Title: The Motility Exam
Author: Eric Peterson, MSIV, BS
Date: N/A
Keywords/Main Subjects: Ocular Motility Exam;
Diagnosis: None
Description of Image:
The ocular motility exam can be a simple yet profoundly important part of the ophthalmic exam. Six extraocular muscles act to move the eye up/down, left/right and intort/excyclotort. These six muscles control the eye in a complex combination of agonist and antagonist cooperation. Interestingly, the extraocular muscles have the densest ratio of motor neurons to muscle fibers of any muscle in the body, thus facilitating the tremendously fine motor control and impeccable alignment of the eyes.
The actions of the eye muscles are most easily assessed by utilizing the H motility pattern as seen in Figure 1. These six cardinal positions along with primary gaze are particularly useful when assessing ocular motility because each cardinal position is primarily obtained by the action of one muscle. For example, elevation of the eye from primary gaze is accomplished by actions of both the superior rectus and the inferior oblique. However, elevation of the abducted eye is primarily accomplished by the superior rectus, while elevation of the adducted eye is primarily accomplished by the inferior oblique. Similarly, depression in abduction isolates the inferior rectus while depression in adduction isolates the superior oblique. Figure 2 demonstrates each of the nine positions with captions describing the muscle(s) being used to obtain that particular position.
When testing motility, assess the eye alignment in primary gaze (consider using the Hirschberg test) and then have the patient move the eyes in an “H” pattern, as shown in Figure 1. Using your finger, a light or a toy, trace an “H” pattern in front of the patient while instructing them to hold their head still. Be sure to alternate between observing the left and right eye in each gaze during the exam.
Format: image
References: none
Faculty Approval by: Griffin Jardine
Copyright Statement: Eric Peterson, ©2016. For further information regarding the rights to this collection, please visit: URL to copyright information page on Moran CORE
Disclosure (Financial or other): none
Hyphema
Home / Basic Ophthalmology Review / Trauma
Title: Hyphema
Author: Bryan Thiel, MS4 – University of Central Florida College of Medicine
Images
Introduction
Hyphema refers to the presence of red blood cells in the anterior chamber of the eye. This is not to be confused with hypopyon which refers to the presence of white blood cells in the same compartment. Hyphemas most commonly arise secondarily to blunt trauma to the globe due to shearing of the small vessels supplying the iris, ciliary body and trabecular meshwork. The severity of the hyphema can either be measured by the depth of settled red blood cells at the base of the anterior chamber or graded on a scale of zero to four as below (Table 1).
Table 1. Grading System of Hyphemas
| Grade 0 or “microhyphema” | Rare cells visible only in slit lamp examination, no layering of cells |
| Grade I | Layering of cells at base of anterior chamber, measuring less than ⅓ the total space |
| Grade II | Layering of cells at the base of the anterior chamber, measuring between ⅓ and ½ the total space |
| Grade III | Layering of cells at the base of the anterior chamber, measuring greater than ½ the total space but not occupying its entirety |
| Grade IV | Layering of cells that occupy the entire total space of the anterior chamber, also called “8-ball” or “black ball” hyphema |
Presentation
Patients will not typically present with complaints of “seeing red” or having “blood in the eye” but rather will have had recent trauma to the globe with symptoms including pain, photophobia and blurred vision. It is imperative to confirm the mechanism of injury, medication use (i.e. anti-platelets, anti-thrombotics) and family history of sickle cell disease or coagulopathy, as these may contribute to the severity of the bleed. A comprehensive eye exam is mandatory in every case of hyphema looking for other sequela of trauma such as ruptured globe, traumatic optic neuropathy or retinal detachments.
Differential
While trauma is the most common source of hyphemas, it is important to keep a broad differential as this symptom may be an early indicator for the following conditions:
Post-surgical hyphema: Surgical manipulation of the anterior structures of the eye may result in bleeding.
- Diabetes: blood in the front of the eye could be a result of ruptured friable vessels that grow on the iris and in the angle in response to long-term retinal ischemia from diabetic retinopathy.
- Intraocular tumors: including, but not limited to, retinoblastomas, iris and ciliary body melanomas and intraocular metastatic malignancies.
- Less commonly: herpetic keratouveitis, rubeosis iridis, juvenile xanthogranuloma, leukemia.
Management
Currently, there is no designated protocol for medical management of hyphemas. Medicinal eye drops such as corticosteroids (e.g. prednisolone acetate) and cycloplegics (cyclopentolate or atropine) are most commonly used. Fibrinolytics, such as aminocaproic acid (ACA) and tranexamic acid (TA), have been extensively discussed in the literature, however sensitivity analyses have demonstrated that these medications minimally reduce the risk of secondary bleeding. Therefore, these drugs have fallen out of favor for treating hyphemas. Patients with hyphemas may develop sudden jumps in their intraocular pressure (IOP) putting them at risk for glaucomatous optic nerve damage and significant discomfort. Therefore, IOP needs to be monitored daily for several days following the injury.
For cases of elevated IOP, first-line therapy includes the use of pressure-reducing medications. Of note, carbonic anhydrase inhibitors like dorzolamide are contraindicated in patients with sickle cell disease or trait, due to their effect on lowering pH which can induce sickling. All patients should be questioned about their sickle cell status and if there is any possibility the patient could have sickle cell disease, hemoglobin analysis testing should be ordered to ascertain disease status of the patient.
Due to the inherent association of hyphema with trauma, inflammation is classically seen in addition to red blood cells. Thus, topical steroids, in addition to cycloplegics, are commonly prescribed to reduce inflammation and the risk of development of iris synechiae (iris scarring to lens or cornea). It is important to keep in mind that once symptoms of inflammation have resolved, topical steroids should be tapered to reduce the risk of steroid-induced glaucoma.
In addition to medication, preventative measures such as placing strict activity restrictions, maintaining an elevated position of the head and using an eye shield are highly recommended to encourage settling of red blood cells and to reduce risk of secondary bleeds. Patients with non-resolving clots, corneal blood staining, or uncontrolled elevated IOPs may benefit from surgical evacuation of the clot or blood from the anterior chamber. All hyphema patients are at lifelong risk of glaucoma due to the damage to the trabecular meshwork from the injury and/or blood and need regular IOP checks for life.
References
- Gharaibeh A, Savage HI, Scherer RW, Goldberg MF, Lindsley K. Medical interventions for traumatic hyphema. The Cochrane database of systematic reviews.2013(12):Cd005431.
- Walton W, Von Hagen S, Grigorian R, Zarbin M. Management of Traumatic Hyphema. Survey of Ophthalmology. 2002;47(4):297-334.
Identifier: Moran_CORE_24622
Ocular Adverse Effects of Systemic Medications: Amiodarone
Home / Basic Ophthalmology Review / Ocular Adverse Effects of Systemic Medications
Title: Ocular Adverse Effects of Systemic Medications: Amiodarone
Author: Emily Ross, 4th Year Medical Student, Indiana University School of Medicine
Location: Med Student Outline > II. Anatomical Approach to Eye Disease > Ocular Adverse Effects of Systemic Medications > 3. Amiodarone
Overview: Amiodarone is a Class III antiarrhythmic medication that slows repolarization of the myocardium which increases the action potential duration and the refractory period, thus slowing the cardiac conduction rate. It is used to treat certain tachyarrhythmias. Long-term use is associated with the following ocular adverse effects.
Corneal verticillata: Also known as vortex keratopathy, occurs in almost all patients who take amiodarone and is related to the dosage of the medication and duration of treatment. It is the most common and most well-known ocular adverse effect of amiodarone. On slit lamp exam, one can see corneal deposits in a whorl-like pattern in the inferior cornea bilaterally (see image 1). The deposits are usually a gray or golden-brown color and accumulate in the basal layer of the corneal epithelium. This is a benign condition and patients usually do not have any visual complaints. Rarely, they report colored rings around lights, haloes and glare which are worse at night. The condition is reversible with discontinuation of the drug. Notably other medications may also cause corneal verticillata including chloroquine and hydroxychloroquine, indomethacin, and phenothiazines and is also seen in Fabry’s disease.
Lens opacities can occur in the anterior cortex of the lens. These are due to pigment deposition and are small and yellowish-white in color. They do not reverse with discontinuation of amiodarone but rarely cause any visual disturbance.
Optic neuropathy is a very rare but serious side effect of long-term amiodarone use. It tends to progress slowly with resulting vision loss. Patients taking amiodarone should have yearly check-ups with an ophthalmologist to monitor for development of optic neuropathy as discontinuing the drug stabilizes the patient’s vision.
References:
Ophthalmology AA of. 2017-2018 Basic and Clinical Science Course Lens and Cataract. S.I.: American Academy of Ophthalmology; 2017.
Ophthalmology AA of. 2017-2018 Basic and Clinical Science Course External Disease and Cornea. S.I.: American Academy of Ophthalmology; 2017.
Graff JM. Verticillata: 54-year-old white male with a known history of atrial fibrillation and hypertension on amiodarone. EyeRounds.org, February 21, 2005; Available from http://www.EyeRounds.org/cases/case29.htm
Mantyjarvi M, Tuppurainen K, Ikaheimo K. Ocular side effects of amiodarone. Survey of Ophthalmology. 1998 Jan-Feb;42(4):360-6.
Giardina E, Zimetbaum P. Monitoring and management of amiodarone side effects. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on September 14, 2017.)
Identifier: Moran_CORE_24549
Bacterial Corneal Ulcer
Home / Basic Ophthalmology Review / Cornea
Name: Paul D Chamberlain, 4th year medical student, Baylor College of Medicine; Dr. Amy Lin, Associate Professor, University of Utah Moran Eye Center.
Topic: Bacterial Corneal Ulcer
Description:
The cornea is the “window” of the eye and consists of five layers (from anterior to posterior): the epithelium, anterior limiting lamina (i.e. Bowman’s layer), stroma, endothelial basement membrane (Descemet’s membrane), and endothelium (image 1). Like ulcers elsewhere in the body, a corneal ulcer is a complete disruption of the epithelial cell layer with an inflammatory response. Unlike other areas of the body, the cornea is dehydrate and avascular which both contribute to its impressive clarity and transparency. The avascular nature of the cornea, however, poses a challenge for the body to fight off infections due to an impaired ability for the immune system to access the infection. For this reason, corneal infections require topical antibiotics and urgent attention.
Corneal ulcers are generally due to bacterial infection, and occur most frequently in patients wearing contact lenses, especially if worn through the night. Bacterial corneal ulcers may also develop secondary to corneal abrasions, blepharitis, or ocular trauma and can be fungal, parasitic, viral or sterile (auto-immune) in addition to bacterial. Patients typically complain of decreased vision, severe pain, redness, or light sensitivity. On physical exam the patient will have injected conjunctiva with a dense collection of inflammatory cells in the cornea called an “infiltrate” (image 2). Marginal corneal ulcers are more commonly multiple and in the periphery of the cornea (where it meets the sclera). These may be due to an immune reaction to staphyloccal toxins from chronic blepharitis (eyelid infection). In severe corneal ulcers, there can be a hypopyon, or dense collection of white blood cells in the anterior chamber (image 4).
Critical to the management of corneal ulcers is assessing the depth of involvement of the cornea and assessing for potential perforation (image 3). This is accomplished with the seidel test. In the seidel test, a small amount of fluorescein dye is placed on the area of ulceration. Using a UV light (either in a slit lamp or with a wood’s lamp) the examiner looks to see if the dye begins to drain down from the ulcer and becomes less bright, indicating that it is being diluted by fluid leaking from the eye. If there is leakage, this is considered “seidel positive.”
Corneal ulcers are an ocular emergency and patients should be referred to an ophthalmologist immediately, as a delay in treatment may result in corneal perforation, endophthalmitis (infection of the entire eye), and/or permanent corneal scarring and subsequent visual impairment. Central corneal ulcers are generally more serious than marginal corneal ulcers. Patients should not be given topical analgesics such as proparacaine for pain relief as continued use horrifically damaging to the eye. To help with pain control, the patient should be prescribed topical parasympatholytics such as atropine or cyclopentolate to reduce ciliary muscle spasm. Culturing the infection on presentation prior to starting treatments also helps guide management in complex or unusual cases.
References:
Leitman MW. (2017). Manual for Eye examination and Diagnosis 9th Ed. Hoboken, NJ: John Wiley & Sons, Inc.
Identifier: Moran_CORE_24538
Preseptal vs Orbital Cellulitis
Home / Basic Ophthalmology Review / Orbit
Name: Paul D Chamberlain, 4th year medical student, Baylor College of Medicine; Reese Feist, Chief Resident, University of Utah Moran Eye Center.
Topic: Preseptal vs Orbital Cellulitis
Terminology and Anatomy
Differentiating orbital from preseptal cellulitis is extraordinarily important given that orbital cellulitis has the potential to cause a compartment syndrome within the eye socket resulting in irreversible vision loss to the affected eye. The orbital septum is a membranous sheath extending from the periosteum of the orbit to the tarsal plate located in the eyelid, and is the key anatomical structure in differentiating preseptal from orbital cellulitis. The orbit (eye socket) is the bony structure in which the globe (eyeball) is housed, and it also contains extraocular muscles, fat, and the blood vessels and nerves that supply these structures. Orbital cellulitis (Image 1), also called post-septal cellulitis, is inflammation of the soft tissues (muscles, fat, and connective tissue) of the orbit most commonly from infection. It is important to remember that in orbital cellulitis, the globe itself is not infected or inflamed. Because the orbit is surrounded by the frontal, ethmoid, and maxillary sinuses, infection often results from extension of a sinus infection.
In comparison, pre-septal cellulitis (Image 2), also known as peri-orbital cellulitis, is an infection of the eyelids and surrounding soft tissues that are anterior to the orbital septum. Both orbital cellulitis and preseptal cellulitis are more common in children, and preseptal cellulitis is much more common that orbital cellulitis.

A patient with orbital cellulitis. Note: orbital cellulitis can take on a variety of clinical manifestations and this image should not be taken as a gold standard to which an examiner compares a patient.
Clinical Manifestations
A number of signs may alert the examiner to the presence of orbital cellulitis (table 1). Patients typicall present with erythema and edema of the eyelids. In orbital cellulitis, they erythema and edema can abruptly stop at the arcus marginalis, where the orbital septum inserts into the periosteum. Preseptal cellulitis typically expands beyond this landmark. Patients may present with eye pain, especially with eye movements due to irritation of inflamed muscles. Partial or complete ophthalmoplegia (inability to move the eye in one or more directions) and associated diplopia (double vision) due to inflammation of extraocular muscles or associated cranial nerves. In orbital cellulitis, inflammation and/or an orbital abscess can displace the globe, often pushing it forward or outward which is called proptosis. The presence of proptosis is a medical emergency to evaluate for compartment syndrome. The eyelids can be swollen and in severe cases swollen shut. Eyelids being swollen shut can be present in either orbital or preseptal cellulitis, and is not as helpful in distinguishing between the two. Visual acuity may be decreased but is often unaffected, and a normal visual acuity should not rule out orbital cellulitis. There is often a history or sinusitis or dental abscess. However, there may be no clear source of inflammation, and it may not even be infectious, as with idiopathic orbital inflammatory syndrome.
Preseptal cellulitis is much more common than orbital cellulitis and also presents with eye pain and erythema of the eyelid and surrounding skin and soft tissue. The inflammation may be great enough to tightly close the eyelids as well (image 3), and they should be opened for a visual inspection. Pre-septal cellulitis does not cause loss of vision and if visual acuity is decreased it is likely because of a poor exam or a more severe infection. Preseptal cellulitis may also arise from sinusitis, but may also arise secondary to local trauma, foreign body, or bug bite (Image 4).

Preseptal cellulitis with mechanical ptosis or droopy eyelid due to edema and erythema of the eyelids. Note that there is no proptosis and the erythema doesn’t abruptly stop at the orbital rim, making it clinically less likely to be orbital cellulitis.

Orbital cellulitis. Note the bullous, edematous conjunctiva (conjunctival chemosis), proptosis and the delineation of swelling around the orbital rim. This patient underwent an urgent lateral canthotomy/cantholysis.
Table 1: Comparison of clinical, historical, and diagnostic characteristics of preseptal and orbital cellulitis.
| Characteristic | Preseptal Cellulitis | Orbital Cellulitis |
| Eye pain | May be present | Yes |
| Eyelid erythema and/or tenderness | Yes | Yes |
| Pain with eye movements | No | May be present |
| Ophthalmoplegia ± diplopia | No | May be present* |
| Proptosis | No | May be present* |
| Vision loss | No | May be present* |
| RAPD | No | May be present* |
| Fever | Usually not present | Usually present |
| Intraocular Pressure (IOP) | Normal | May be elevated* |
| Resistance to Retropulsion | None | Present* |
| History of sinusitis | May be present, but often not | Present more often than not |
| CT or MRI imaging | Shows inflammation only anterior to the orbital septum | Show post-septal involvement of the inflammation. |
| Blood Cultures | Very rarely has bacteremia | Bacteremia may be present |
| *Emergent signs and symptoms that might warrant immediate lateral canthotomy/cantholysis | ||
Work-up and Treatment
As previously discussed, the first step is to determine whether a patient has orbital cellulitis, which requires orbital imaging, admission, blood cultures and IV antibiotics. Begin with a thorough ophthalmologic exam, with particular attention to visual acuity, pupillary testing for a relative afferent pupillary defect (RAPD), intraocular pressure, and assessment of proptosis and eye motility. In cases of question, Computed tomography (CT) with and without contrast of the orbits and sinuses should be ordered to look for evidence of post-septal involvement. If a diagnosis of orbital cellulitis is made, the patient needs to be immediately assessed monitored for signs of compartment syndrome and optic neuropathy which would warrant an emergent lateral canthotomy/cantholysis. This procedure allows for anterior expansion of orbital contents which relieves pressure within the orbit in restores blood flow to those structures. Even after initiating antibiotics the swelling may increase for the first 24-48 hours, so frequent re-evaluation is warranted. Immediate surgery may be indicated if there is evidence of a subperiosteal abscess, orbital abscess, or extension of the infection into the cranium. Consider consulting an otolaryngologist for management of sinus disease.
Patients whose history and examination are consistent with preseptal cellulitis without symptoms of orbital cellulitis may be treated as an outpatient with oral antibiotics. Patients with preseptal cellulitis who are appropriately treated typically recover completely without any permanent sequelae. If treated appropriately, patients with orbital cellulitis also often have good outcomes. However, failure to diagnose and treat orbital cellulitis in a timely manner may result in permanent vision loss. An ophthalmologist should be consulted in all cases of orbital or preseptal cellulitis. However, assessing for vision threatening orbital cellulitis should not be postponed until an ophthalmologist is available as an irreversible ischemic optic neuropathy can occur in less than 90 minutes.
References:
Amin N, Syed I, Osborne S. Assessment and management of orbital cellulitis. British Journal of Hospital Medicine. 2016; 77(4):216-20.
Hauser A, Fogarasi S. Periorbital and orbital cellulitis. Pediatrics in Review. 2010; 31(6):242-9.
Meara DJ. Sinonasal disease and orbital cellulitis in children. Oral Maxillofacial Surgery Clinics of North America. 2012; 24(3):487-96.
Rashed F, Cannon A, Heaton PA, Paul SP. Diagnosis, management and treatment of orbital and periorbital cellulitis in children. Emergency Nurse. 2016; (24(1):30-5.
Identifier: Moran_CORE_24521
Dry Eye Disease
Home / Basic Ophthalmology Review / Lids/Lashes
Title: Dry Eye Disease
Author: Tanner Ferguson, 4th year medical student, University of South Dakota Sanford School of Medicine
Description: Dry eye disease is a very common condition and is one of the most common causes of eye discomfort in the general population. Dry eye disease is typically split into two categories: evaporative dry eye and decreased aqueous production1. Recently, increased interest in dry eye disease and the importance of the ocular surface has paved the way to newer treatment modalities. Being equipped with the awareness of the signs and symptoms will help clinicians manage these patients appropriately. It is important to recognize the risk factors, common signs and symptoms of dry eye disease so that patients can be appropriately treated.
Patients with dry eye can present with a variety of signs and symptoms. Symptoms of fluctuations in vision, discomfort, burning or a foreign body sensation are common2. Patients may demonstrate conjunctival injection and eyelid swelling/inflammation, or blepharitis.
Firstly, take a careful history as environmental factors and diet can significantly contribute to dry eye disease2. Ask the patient about the use of ceiling/direct fans, humidifiers, water intake, contact lens wear, time outdoors, direct sun exposure and daily activities. Patients who work long hours on near tasks such as a computer frequently blink less and are subsequently at-risk for dry eye3,4. Some medications may also contribute to dry eyes, such as antihistamines, pain relievers, certain anti-hypertensives, decongestants, hormones, antidepressants, antipsychotics, chemotherapeutics and acne medications2. Diet is another significant contributor; studies suggest that patients who supplement with omega-3-based vitamins report less dry-eye related symptoms.5
When a patient presents with an irritated red eye, it is important to be cognizant of dry eye disease and gather a full history to prevent unnecessary treatments with extensive or prolonged antibiotic regiments. If you suspect a patient is suffering from dry eye disease, encourage them to seek out an eye care provider and manage any modifiable risk factors in the meantime.
References:
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75-92.
- Lemp MA. Advances in understanding and managing dry eye disease. American Journal of Ophthalmology. 2008;146(3):350-356. doi:10.1016/j.ajo.2008.05.016.
- Tsubota K, Nakamori K. Dry Eyes and Video Display Terminals. N Engl J Med. 1993;328(8):584-584. doi:10.1056/NEJM199302253280817.
- Tsubota K. Tear dynamics and dry eye. Progress in Retinal and Eye Research. 1998;17(4):565-596.
- Korb DR, Blackie CA, Finnemore VM, Douglass T. Effect of Using a Combination of Lid Wipes, Eye Drops, and Omega-3 Supplements on Meibomian Gland Functionality in Patients With Lipid Deficient/Evaporative Dry Eye. Cornea. 2015;34(4):407-412. doi:10.1097/ICO.0000000000000366.
Identifier: Moran_CORE_24492
3rd Nerve Palsy
Home / Basic Ophthalmology Review / Extraocular Muscles / Motility
Title: 3rd Nerve Palsy
Author: Patrick Commiskey, 4th Year Medical Student, University of Michigan Medical School
Description: The 3rd cranial nerve (CN III), or oculomotor nerve, is a motor nerve responsible for many eye-related functions. The third cranial nerve innervates four of the six extraocular muscles: medial rectus, superior rectus, inferior rectus, and inferior oblique. Parasympathetic nerve fibers originating from the Edinger-Westphal nucleus travel circumferentially with CN III to the pupil. causing pupillary constriction when activated. The levator palpebrae superioris muscle, which is the primary muscle in elevating the eyelid is innervated by the superior division of CN III.
A complete lesion of CN III would result in ipsilateral ptosis (droopy eyelid) and a “down and out” eye due to denervation of most extraocular muscles leaving the lateral rectus (innervated by CN VI, abducts) and superior oblique (innervated by CN IV, depresses) unopposed. A dilated pupil (mydriasis) resulting from disruption of the parasympathetic fibers are the result of denervation of the sphincter pupillae.
Understanding the anatomy of CN III is important to deciphering important the etiology of a CN III palsy. Most lesions of the 3rd cranial nerve are from vascular insults causing ischemia and can be attributed to known vascular risk factors such as diabetes or hypertension. These presentations typically have ptosis, a down-and-out eye but a normal pupil. However, a compressive or space-occupying lesion such as an aneurysm or tumor would cause disruption of the parasympathetic fibers because of their superficial location on the outside of CN III. In fact, an acutely “blown” pupil in conjunction with the above mentioned ptosis and motility deficits may be an ominous sign of a growing aneurysm. Retrospective review studies have shown that we cannot completely rely upon the pupil to differentiate vascular from compressive causes because numerous exceptions have occurred. Therefore, a “blown” pupil is treated emergently and requires neuro-imaging, but the lack of this sign does not exclude an acute space-occupying pathology.
Additional etiologies of 3rd nerve palsy to consider include Myasthenia Gravis, Thyroid disease, internuclear ophthalmoplegia, orbital tumor or pseudotumor, and temporal arteritis (giant cell arteritis).
References:
- Jacobson DM , BrosteSK. Early progression of ophthalmoplegia in patients with ischemic oculomotor nerve palsies.Arch Ophthalmol.1995;113(12):1535–1537.
- Trobe JD . Managing oculomotor nerve palsy.Arch Ophthalmol.1998;116(6):798.
Identifier: Moran_CORE_ID: 24487
Leukocoria (in children)
Home / Basic Ophthalmology Review / Pupillary Exam
Title: Leukocoria (in children)
Author: Spencer Fuller, MSIV – UC San Diego School of Medicine, MPH
Definition: In contrast to the normal red reflex, leukocoria is defined as a yellow, pale, white, or otherwise abnormal reflection of light observed in the pupil of one or both eyes.
Presentation: The asymmetric “white pupil” is either caught by family members (often in photos with asymmetric red reflexes from the flash) or incidentally by a practitioner on routine direct ophthalmoscopy. Every well child check—especially the newborn baby check in the nursery—should include an examination of the pupils. Patients in whom leukocoria is observed should be promptly referred to an ophthalmic specialist for diagnosis and management.
Differential Diagnosis:
The differential diagnosis of leukocoria in children is broad and includes hereditary, developmental, inflammatory, neoplastic and miscellaneous conditions [1]:
- Strabismus – a common pediatric problem, ocular misalignments can cause asymmetric red reflex testing [see American Academy of Pediatrics (AAP) insert below]. Prompt referral of strabismus to a pediatric ophthalmologist is important to prevent amblyopia or poor visual development in the misaligned eye.
- Anisometropia – difference in refractive errors or prescriptions can cause an asymmetric red reflex where on eye is dimmer than the other—otherwise known as the “Bruckner test.”
- Retinoblastoma – the most common pediatric ocular malignancy, occurs primarily in children aged 18-24 months of age but can be much younger in those at higher risk. Retinoblastoma has been estimated to account for as much as 50% of leukocoria cases in United States.
This patient with leukocoria eventually was diagnosed with Retinoblastoma and underwent enucleation.
Jordan, Michael (2014). 2 year Old with Leukocoria. Moran Eye Center Grand Rounds http://morancore.utah.edu/section-06-pediatric-ophthalmology-and-strabismus/case-2-year-old-with-leukocoria/
- Congenital cataract – develops from numerous processes (i.e. infectious, metabolic, genetic) and can be either unilateral or bilateral. These can cause irreversible vision loss within a matter of weeks to months—prompt recognition, referral and surgical treatment is vital.
- Retinopathy of prematurity (ROP) – the most common cause of blindness in children in the United States, ROP occurs in premature (gestational age < 30 weeks) or underweight (< 1500 grams) babies as a result of abnormal retinal vessel development. Retinopathy develops due to irregular growth of retinal blood vessels that can lead to bilateral blinding retinal detachments. Thanks to an increased understanding of supplemental oxygen regulation in the NICU as well as ROP progression most cases are caught and treated. ROP patients are regularly followed by a pediatric ophthalmologist or retinal specialist.
- Coats’ disease – abnormal development of temporal retinal vessels that is ten times more common in males, primarily affects only one eye and usually seen in patients younger than 8 years of age.
- Persistent hyperplastic primary vitreous – usually unilateral and results from failure of regression of embryological vitreous and blood vessels and is often associated with a cataract or retrolental fibroplasia.
- Inflammation (i.e. ocular toxocariasis, congenital CMV) – any intraocular inflammatory process can cause leukocoria from an accumulation of inflammatory debris, especially if the vitreous is involved.
- Coloboma – results from failure of the developing eye to complete circumferential fusion of the developing nasal and temporal poles. Ocular colobomas occur inferiorly in the eye and can involve the optic nerve, retina, lens and/or iris. From a primary care perspective, an iris coloboma (an inferonasal defect in the iris) is most easily recognized as it is the most anterior. For such patients, referral to a pediatric ophthalmologist for a dilated eye exam is important to assess whether the coloboma has affected deeper structures within the eye.
- Familial exudative vitreoretinopathy (FEVR) – has features that overlap with ROP but with different patient characteristics (i.e. not premature) and disease time course. The hallmark features are peripheral zones of avascular retina that are prone to abnormal blood vessel formation, leakage and exudate formation, and vitreous traction that often results in retinal detachments.
- Retinal detachment – though rare in the pediatric population, there is increased prevalence of retinal detachments in patients with ROP, high myopia (near-sightedness), and other ophthalmologic problems like FEVR.
Diagnosis: The following modalities may be used by ophthalmic specialists to determine the cause of leukocoria:
- Direct ophthalmoscopy
- Slit lamp biomicroscopy and indirect ophthalmoscopy
- Retinal fundus photography
- Fluorescein Angiography
- Orbital/Head imaging often aids to narrow the differential diagnosis
- B-scan ultrasonography
- Optical coherence tomography (OCT)
- MRI of the head and orbits (CT scans are avoided in case of malignancy to avoid radiation exposure)
Management: Management of leukocoria varies and is dependent on the specific cause. Though retinoblastoma is the most feared cause of the leukocoria, many referrals for an asymmetric or abnormal red reflex turn out to be nothing. Nonetheless, a timely referral for a dilated eye exam is still vital. In the infant to toddler age range it is important to ask about a family history of retinoblastoma or congenital cataracts along with taking a careful birth and delivery history. In the case of a congenital cataract (which may be unilateral or bilateral), the timing of cataract surgery is amongst the most important factors in eventual visual outcomes. Unilateral cataracts are ideally removed at six weeks of ages and bilateral cataracts by eight to ten weeks. Missing this window can cause irreversible deprivational amblyopia, or permanent blindness in one or both eyes.

From: American Academy of Pediatrics. (2008). Red reflex examination in neonates, infants, and children. Pediatrics, 122(6), 1401-1404.
See Red handout from the American Academy of Pediatrics: http://pediatrics.aappublications.org/content/122/6/1401
References:
- Stagg, B., Ambati, BK. et al. (2014) Diagnostic Ophthalmology. Amirsys Publishing, Inc., Manitoba, Canada.
- Shields, J. A., Shields, C. L. (2008). Retinoblastoma: Introduction, Genetics, Clinical Features, Classification. In Intraocular Tumors: An Atlas and Textbook (pp. 293-318). Lippincott Williams & Wilkins, Philadelphia, PA.
- Shields, J. A., Shields, C. L. (2008). Retinoblastoma: Diagnostic Approaches. In Intraocular Tumors: An Atlas and Textbook (pp. 319-326). Lippincott Williams & Wilkins, Philadelphia, PA.
- Shields, J. A., Shields, C. L. (2008). Lesions That Can Simulate Retinoblastoma. In Intraocular Tumors: An Atlas and Textbook (pp. 353-366). Lippincott Williams & Wilkins, Philadelphia, PA.
- Balmer, A., & Munier, F. (1999). Leukokoria in a child: emergency and challenge. Klinische Monatsblatter Fur Augenheilkunde, 214(5), 332-335.
- Haider, S., Qureshi, W., & Ali, A. (2008). Leukocoria in children. Journal of pediatric ophthalmology and strabismus, 45(3), 179-180.
- American Academy of Pediatrics. (2008). Red reflex examination in neonates, infants, and children. Pediatrics, 122(6), 1401-1404.
Identifier: Moran_CORE_24126