Duane Syndrome
Home / Pediatric Ophthalmology and Strabismus / Special Forms of Strabismus
Title: Duane Syndrome
Author: Kerri McInnis-Smith, 4th year medical student, Mayo Clinic
Photographer: Dr. Marielle Young
Date: 7/14/2022
Keywords/Main Subjects: Strabismus, Duane Syndrome, Esotropia
Diagnosis: Duane Syndrome
Images or video:
Description of Case: Duane Syndrome, also known as Duane Retraction Syndrome (DRS), is a form of childhood strabismus characterized by globe retraction and palpebral fissure narrowing on attempted adduction.
Epidemiology: The prevalence of DRS in the general population is relatively low, affecting around 1/1000 individuals and accounting for <5% of all strabismus cases.1 Only one eye is involved in most cases, although up to 20% of affected patients may have bilateral involvement.2 For reasons that remain unclear, female patients are more commonly affected (60%) by DRS than male patients (40%) and the left eye is involved more often than the right eye.1,2 There is osme thought that the unilateral, left-sided, and female predominance could be due to the asymmetry in the thoracic anatomy and thrombosis risk factors.3
Pathophysiology: DRS is caused by abnormal (and sometimes absent) development of the abducens nerve (CN VI) between weeks 4-8 of embryological development. Occasionally, the lateral rectus muscle may receive aberrant innervation from the oculomotor nerve (CN III), contributing to concurrent horizontal recti action and subsequent globe retraction.4 Most cases (70%) of DRS are isolated to the below ocular findings, while around 30% are associated with additional ophthalmologic or systemic abnormalities. Some associated conditions include:2,5,6
- Okihiro’s syndrome: DRS + radial ray defects
- Wildervanck syndrome: DRS + Klippel-Feil anomaly + deafness
- Moebius syndrome: DRS + congenital facial palsy
- Hold-Oram syndrome: DRS + abnormalities of upper limbs and heart
- Morning Glory syndrome: abnormalities of optic disc
- Goldenhar syndrome: abnormalities eye, ear and spine
Risk factors: Although DRS occurs spontaneously in approximately 90% of isolated cases, around 10% of isolated cases are inherited.7 The only known risk factor for development of the condition is an affected biological parent. Various genes have been implicated in the inheritance of DRS, including mutations at locus 8q13 and in CHN1 on chromosome 2. Both autosomal dominant and autosomal recessive inheritance patterns have been demonstrated.2,5
Signs and symptoms:
- Complete or partial absence of abduction and/or adduction
- Retraction of globe on attempted adduction
- Narrowing of palpebral fissure on attempted adduction (induced ptosis)
- Abnormal head position (to compensate for duction deficit and maintain binocular single vision)
- Upshoots or downshoots (43% of cases2): affected eye deviates up/down with attempted adduction
- May occur secondary to mechanical effect (tight fibrotic muscles) or innervational anomalies2
Diagnosis: Diagnosis of DRS is typically made on clinical grounds alone, with additional imaging usually not necessary. Genetic testing may be pursued if familial inheritance is suspected.
Subtypes: Multiple criteria have been proposed to classify DRS according to clinical signs and symptoms. The most popular classification system, proposed by Huber et al, consists of 3 distinct subtypes:1
- Type I DRS (75-80%): mainly defective abduction, with normal or minimally defective adduction
- Esotropia in primary gaze compensatory head turn toward involved side
- Type II DRS (5-10%): mainly defective adduction, with normal or minimally defective abduction
- Exotropia in primary gaze compensatory head turn toward uninvolved side
- Type III DRS (10-20%): defective in both abduction and adduction
Individuals can also be sub-grouped according to their deviation in primary position, including esotropic DRS (eso-DRS), exotropic DRS (exo-DRS), and orthotropic DRS (exo-DRS).1 These two systems of classifying DRS can be helpful in the decision of whether to manage conservatively or surgically.
Differential diagnosis:5,7
- Abducens nerve palsy
- Congenital esotropia
- Brown Syndrome
- Marcus Gunn Jaw Winking Syndrome
- One of the associated systemic conditions mentioned above (Okihiro’s syndrome, Goldenhar syndrome, Wildervanck syndrome, Moebius syndrome, Holt-Oram syndrome, Morning Glory syndrome)
Management:
- Non-surgical: Not all individuals with DRS require surgical intervention. Conservative measures, such as observation, refractive correction, or prism glasses to improve abnormal head position are often sufficient to manage symptoms. Young patients should undergo repeat ophthalmology exams to assess for amblyopia. However, once a patient is not at a significant risk of developing amblyopia (around age 10), exams can occur less frequently.2
- Surgical: A subset of patients with DRS (estimated around 41%1) will progress to requiring surgical intervention. There are four generally accepted indications for which extraocular muscle surgery should be considered:1
- Significant abnormal head posture
- Significant deviation in primary position
- Severely abnormal eyelid position (retraction and narrowing of palpebral fissure)
- Significant upshoot or downshoot during adduction
The exact surgical approach is dependent on the patient’s symptoms, deviation in primary position, and specific duction deficits, but often consists of medial or lateral rectus recession and/or transposition of one or two vertical rectus muscles. Globe retraction can be improved via recessions of the co-contracting horizontal recti muscles.1
Potential complications: Although isolated DRS is not associated with severe complications, up to 10% of patients may develop amblyopia, especially without regular ophthalmologic exams.1
References:
- Gaballah KA. Treatment modalities in Duane’s Retraction Syndrome. Int J Ophthalmol. 2020 Feb 18;13(2):278–83.
- Kekunnaya R, Negalur M. Duane retraction syndrome: causes, effects and management strategies. Clin Ophthalmol. 2017 Oct;Volume 11:1917–30.
- Parsa CF, Robert MP. Thromboembolism and Congenital Malformations: From Duane Syndrome to Thalidomide Embryopathy. JAMA Ophthalmol. 2013 Apr 1;131(4):439.
- Hoyt W, Nachtigäller H. Anomalies of ocular motor nerves: Neuroanatomic correlates of paradoxical innervation in Duane’s syndrome and related congenital ocular motor disorders. Am J Ophthalmol. 1965 Sep;60(3):443–8.
- Graeber CP, Hunter DG, Engle EC. The Genetic Basis of Incomitant Strabismus: Consolidation of the Current Knowledge of the Genetic Foundations of Disease. Semin Ophthalmol. 2013 Sep;28(5–6):427–37.
- Kirkham TH. Duane’s syndrome and familial perceptive deafness. Br J Ophthalmol. 1969 May 1;53(5):335–9.
- Gaur N, Sharma P. Management of Duane retraction syndrome: A simplified approach. Indian J Ophthalmol. 2019;67(1):16.
Faculty Approval by: Marielle Young, MD
Identifier: Moran_CORE_127212
Copyright Kerri McInnis-Smith, ©2024. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
Fundus Photography, Fluorescein Angiography and Optical Coherence Tomography of Bilateral Exudative Detachments in a Pediatric Patient
Home / Pediatric Ophthalmology and Strabismus / Disorders of the Retina and Vitreous
Title: Fundus Photography, Fluorescein Angiography and Optical Coherence Tomography of Bilateral Exudative Detachments in a Pediatric Patient
Author: Olaoluwa Omotowa, MPH, Nnana Amakiri, MD, Marcus Altman, MD, Theresa Long, MD
Keywords/Main Subjects: Bilateral Exudative Retinal Detachments
Diagnosis: Bilateral Exudative Retinal Detachments
- Figure 1: Optical Coherence Tomography of Right Eye demonstrating inferior serous retinal detachment sparing the macula with optic disc edema.
- Figure 2: Optical Coherence Tomography of Left Eye demonstrating serous retinal detachment with macular involvement and optic disc edema.
- Figure 3: Color Fundus demonstrating Bilateral Serous Retinal Detachments with peripapillary cotton wool spots and full detachment of the macula OS. Elschnig spots in periphery OU.
- Figure 4: Fluorescein Angiography demonstrating patchy choroidal filling with scattered, nonspecific, hypofluorescent lesions with late hyperfluorescent lesions OU.
Description of Case:
A 4-year-old girl with a history of chronic hypertension, albinism, hypothyroidism, and complicated delivery requiring an extended NICU stay was referred to our facility with progressive ataxia, facial weakness, and loss of appetite. She was afebrile without new rashes or constitutional symptoms. The patient’s medical history included a seizure during NICU stay and a diagnosis of Bell’s Palsy at age 1. Additionally, she had a history of being diffusely edematous and hypertensive while in the NICU, which led to treatment with anti-hypertensive medications. At home her systolic blood pressures were reportedly in the 130-140s. Initial CT at an outside hospital revealed ventriculomegaly and brainstem glioma prompting urgent referral to our facility and neurosurgical consultation with administration of 4mg dexamethasone IV.
During her current presentation, she developed bilateral facial weakness, including ptosis related to Bell’s Palsy, along with her progressive ataxic gait, right ear pain, decreased appetite, and occasional emesis. On arrival, she had age-appropriate vital signs (94/58) and normal mental status.
Her neurological status deteriorated, necessitating an MRI scan. While lying flat in the MRI scanner, she became hypertensive with SBP in 180s. The scan revealed worsening ventriculomegaly, longitudinally extensive transverse myelitis, bilateral retinal hemorrhages, and nonenhancing T2/FLAIR signal in various brain regions. She received a total of 18 mg labetalol and ultimately required nicardipine drip 1.5 mcg/kg/hr normalizing her blood pressure to 138/100. She was intubated in MRI and taken for emergent EVD placement with neurosurgery. She tolerated the procedure well without complications and was subsequently weaned off her nicardipine drip. Given the ocular findings on imaging, ophthalmology was promptly consulted.
Ophthalmic examination revealed no light perception in both eyes both of which were soft to palpation. She had mild esophoria and no afferent pupillary defect though was on miotics. Her fundus examination revealed bilateral exudative retinal detachments, tortuous vessels, and diffuse serous detachments with a tigroid appearance and diffuse creamy infiltrates. Further evaluation, including ocular ultrasonography and fluorescein angiography, was performed to ascertain the underlying cause.
A comprehensive workup, including laboratory investigations, lumbar puncture, whole-genome sequencing, and whole body imaging was initiated to identify the etiology of the bilateral exudative retinal detachments. Laboratory results indicated AQP4-IgG, Adams13, MOG Ab IgG, D-dimer, Haptoglobin, RPR, TSH, C3, C4 CRP, and ESR were within normal limits. RCIGM revealed no genomic variants. Anti-CFH Autoantibody, HSV IgG/IgM, B Burgdorferi IgM & IgG, Quant Gold, Cat Scratch IgG & IgM, ANCA, HIV, Treponema, meningitis/encephalitis panel, oligoclonal bands, CSF flow and cytology were negative. LDH (740), platelets (316), IgG (333), IgG synthesis (14.5), and Von Willebrand Ag (150%) were all elevated. The patient’s clinical and diagnostic findings led to a diagnosis of hypertensive chorioretinopathy as the most likely cause.
Patient was scheduled for close follow up 2 weeks after her initial diagnosis. After not making it to this appointment she was rescheduled with her hometown ophthalmologist. These visits demonstrated gradual resolution of subretinal fluid and exudates. Serial optical coherence tomography scans and dilated examinations demonstrated reattachment of the retina in both eyes, with the patient’s visual acuity improving, although some residual impairment remained due to macular involvement. She was 20/300 in her right eye and 20/200 in her left.
Summary of the Case:
- Bilateral exudative retinal detachments in pediatric patients present diagnostic challenges due to their rarity and various potential etiologies.
- The causes can be classified as inflammatory, infectious, or neoplastic, including conditions like familial exudative vitreoretinopathy, hemolytic uremic syndrome, choroidal lymphoma, hemangioma, and metastases.
- The presented case highlights the importance of considering rare causes, such as malignant hypertension, in patients with bilateral exudative retinal detachments. Prompt control of blood pressure played a crucial role in the resolution of fundus findings.
- This case emphasizes the significance of thorough evaluation and heightened awareness among eyecare providers when encountering pediatric patients with bilateral exudative retinal detachments. Timely diagnosis and management are crucial to prevent irreversible vision loss.
- Hypertensive chorioretinopathy should be considered as a possible etiology in cases of acute on chronic hypertension presenting with retinal detachments.
References:
- Yoshida, K., Hasegawa, D., Takusagawa, A., Kato, I., Ogawa, C., Echizen, N., … & Manabe, A. (2010). Bullous exudative retinal detachment due to infiltration of leukemic cells in a child with acute lymphoblastic leukemia. International journal of hematology, 92, 535-537.
- Rosecan, L. R., Laskin, O. L., Kalman, C. M., Haik, B. G., & Ellsworth, R. M. (1986). Antiviral therapy with ganciclovir for cytomegalovirus retinitis and bilateral exudative retinal detachments in an immunocompromised child. Ophthalmology, 93(11), 1401-1407.
- Navarrete, A., Jaouni, T., & Amer, R. (2023). Total exudative retinal detachment in a child with pars planitis-a challenging case with optimistic results. Journal of Ophthalmic Inflammation and Infection, 13(1), 1-4.
- Otuka, O. A. I., Eweputanna, L. I., Okoronkwo, N. C., & Kalu, A. (2021). Bilateral Exudative Retinal Detachment in a Young Patient with Chronic Renal Failure. International Medical Case Reports Journal, 139-144.
- Khaja, W. A., Pogrebniak, A. E., & Bolling, J. P. (2015). Combined orbital proptosis and exudative retinal detachment as initial manifestations of acute myeloid leukemia. Journal of American Association for Pediatric Ophthalmology and Strabismus, 19(5), 479-
- Shukla UV, Gupta A, Tripathy K. Exudative Retinal Detachment. 2023 Feb 22. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 36944005.
Faculty Approval by: Theresa Long, MD
Copyright statement: Olaoluwa Omotowa, ©2023. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
Intraocular Pressure Fluctuation in a Patient with Pseudoexfoliation Glaucoma
Title: Intraocular Pressure Fluctuation in a Patient with Pseudoexfoliation Glaucoma
Authors: Tara Gallant, California Northstate University College of Medicine, MD Class of 2024; Barbara Wirostko, MD
Date: September 2023
Keywords/Main Subjects: Pseudoexfoliation Syndrome, Pseudoexfoliation Glaucoma, Intraocular Pressure Fluctuations, Home iCare
Introduction:
Pseudoexfoliation syndrome (XFS) results from abnormal fibrillar extracellular material accumulation in ocular tissues, including all structures of the anterior segment, the conjunctiva, and orbital structures.1 XFS is one of the most common causes of open-angle glaucoma. Pseudoexfoliation glaucoma (XFG) typically presents with a higher maximum and mean IOP at the time of diagnosis, as well as a wider range of IOP fluctuation, compared with primary open angle glaucoma (POAG). It is associated with a higher risk of progressive vision loss than POAG, more rapidly developing cataracts, phacodenesis, lens subluxation, and retinal vascular events.1,2 XFS manifestations are age-related and estimated to be present in 10-20% of the general population above the age of 60 depending upon the geographic location.2 Patients with XFS are thought to have a ten-fold higher risk of developing glaucoma than the general population.2 On dilated clinical exam, XFS can be identified by the presence of white deposits on the anterior lens surface and pupillary margins. Ultrasound biomicroscopy often reveals zonular weakness, a thickened lens, a narrow anterior chamber, and occludable angles.2 Systemic diseases with an increased incidence in patients with XFS include chronic obstructive pulmonary disease, inguinal hernias, pelvic organ prolapse, obstructive sleep apnea, and atrial fibrillation.3
Case Presentation:
Our patient is an 81-year-old female with severe stage capsular pseudoexfoliation glaucoma in both eyes. Five years ago, she underwent Phaco CyPASS surgeries in both eyes. Approximately two years ago, the patient had a XEN gel stent with mitomycin C/Ologen placed in the right eye. Her current ocular medications include Cosopt BID OU and Vyzulta QHS OU. Other ocular conditions include dry eye syndrome and meibomitis. She has a history of pelvic organ prolapse, and her mother was also diagnosed with glaucoma and pelvic organ prolapse.
At her most recent visit, visual acuity was 20/30 OD and 20/25 OS, with applanation tonometry readings of 8 OD and 12 OS. Slit lamp exam was notable for pseudoexfoliation material on the anterior aspect of the lens capsule with no transillumination defects bilaterally. She was also noted to have phacodenesis OD. Her right optic nerve had temporal pallor and a cup/disc ratio of 0.95, and her left optic nerve had an infratemporal notch with a cup/disc ratio of 0.6. Other exam findings included trace meibomian gland dysfunction OU, trace hordeolum OU, and well-centered posterior chamber intraocular lenses bilaterally. Optic nerve OCT scans demonstrated diffuse temporal thinning OD and infratemporal thinning OS, with no progression of glaucomatous damage over the past year and a half in either eye when compared to prior scans.
Two and a half years ago, the patient was assigned an iCare HOME to determine if she was experiencing intraocular pressure (IOP) fluctuations that could help explain the severe and progressive glaucomatous damage to her optic nerves, particularly in her right eye. A summary of the results is shown in Figure 1 below. The measurements taken revealed higher overall IOP in the right eye outside of normal clinic hours, as well as increased fluctuation in pressures when compared to the left eye. The highest and lowest measurements taken are shown in Table 1 below.
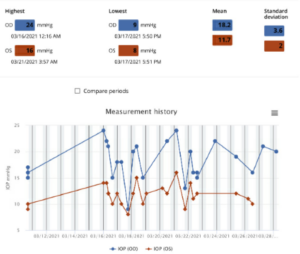
Figure 1: Summary of the results of iCare Home measurements taken three months prior to the glaucoma stent procedure OD. All the measurements were “excellent” in quality. Average IOP and variability of IOP was higher in the right eye, which had more glaucomatous damage compared to the left eye. Both average IOP and variability of IOP in OD were higher on these iCare measurements than on clinical measurements around the same time.
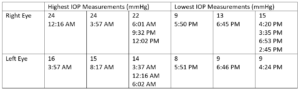
Table 1: Highest and lowest IOP measurements taken in each eye while the patient had the iCare HOME and the corresponding times at which the measurements were taken three months prior to the glaucoma stent procedure OD. Notably, the highest IOP measurements were recorded overnight and in the morning, whereas the lowest IOP measurements were recorded in the afternoon and early evening.
The decision was made to perform additional surgery in the right eye three months after those initial iCare measurements were taken to better control IOP fluctuations, as the patient had pericentral visual field loss OD (Figure 2) and diffuse optic nerve thinning temporally OD (Figure 3).
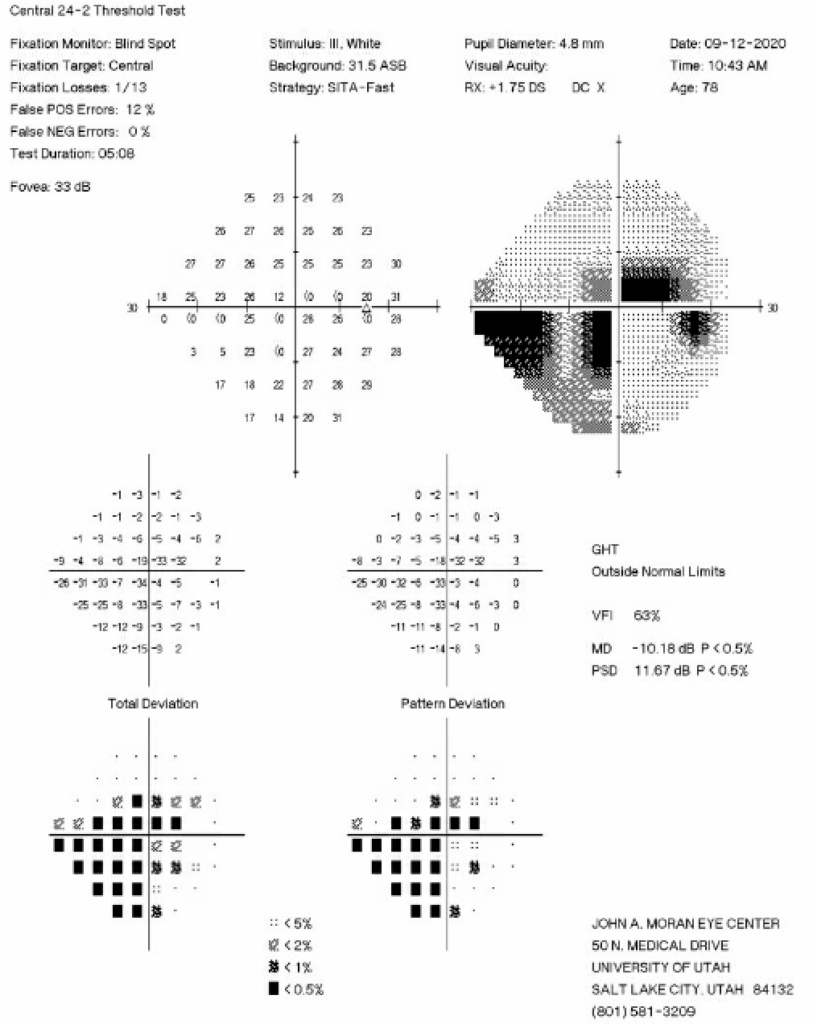
Figure 2: 24-2 Humphrey Visual Field test from six months prior to the initial iCare HOME measurements showing an inferior nasal step and pericentral visual field loss in the right eye.
Nine months after the XEN gel stent with mitomycin C/Ologen was placed in the right eye, the patient was asked to repeat the iCare measurements. A summary of the results is shown in Figure 4 below.

Figure 4: Summary of the results of iCare HOME measurements taken nine months after the glaucoma stent procedure OD. All the measurements were “excellent” in quality. Average IOP and variability of IOP in the right eye were now both lower compared to the iCare HOME measurements from one year prior.
Discussion:
IOP varies both daily and hourly, and clinicians are not able to fully understand patients’ IOP when only capturing measurements at office visits. In a study conducted at the Wilmer Eye Institute, it was found that mean IOP was slightly lower by home tonometry readings than by clinic readings alone, while IOP fluctuation and IOP spikes were significantly higher than those measured in clinic.4 Additionally, those researchers found that mean daily measurements exceeded the recent clinic maximum IOP in 44% of patients, and that mean daily measurements by home iCare were greater than any historic IOP measured in clinic in 13% of patients.4 Furthermore, the peak home IOP occurred outside of the typical 8:00 am – 5:00 pm office hours on half of the days.4 Diurnal IOP fluctuations have been documented dating back several decades. In one study analyzing 2272 diurnal curves of IOP measurements, 41% of the peaks were found at the early morning IOP measurement, while 24% of peaks were found at the second, mid-morning measurement.5 Another study showed this pattern of nocturnal IOP elevation occurs independently of central corneal thickness, corneal hysteresis, and corneal resistance factor.6
Mean IOP and diurnal fluctuation in IOP are typically higher in XFG than in POAG, and reduction of these measures has been documented to be more effective in XFG than in POAG in preventing visual field damage.7 In our patient, implanting a XEN gel stent with mitomycin C/Ologen in the eye with significant glaucoma damage lowered her average home iCare reading in that eye from 18.2 to 9.5, and the standard deviation of IOP measures decreased from 3.6 to 1.0. With these significantly improved eye pressures, RNFL thickness and visual fields have remained relatively stable for the past two years since her surgery. This case demonstrates the importance of understanding how IOP varies throughout the day, and the efficacy of surgical intervention on minimizing both fluctuations in these measurements and glaucomatous changes in the eye.
References:
-
- Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45(4):265-315. doi:10.1016/s0039-6257(00)00196-x
- Yüksel N, Yılmaz Tuğan B. Pseudoexfoliation Glaucoma: Clinical Presentation and Therapeutic Options. Turk J Ophthalmol. 2023;53(4):247-256. doi:10.4274/tjo.galenos.2023.76300
- Pompoco CJ, Curtin K, Taylor S, et al. Summary of Utah Project on Exfoliation Syndrome (UPEXS): using a large database to identify systemic comorbidities. BMJ Open Ophthalmol. 2021;6(1):e000803. doi:10.1136/bmjophth-2021-000803
- McGlumphy EJ, Mihailovic A, Ramulu PY, Johnson TV. Home Self-tonometry Trials Compared with Clinic Tonometry in Patients with Glaucoma. Ophthalmol Glaucoma. 2021;4(6):569-580. doi:10.1016/j.ogla.2021.03.017
- David R, Zangwill L, Briscoe D, Dagan M, Yagev R, Yassur Y. Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br J Ophthalmol. 1992;76(5):280-283.
- Bagga H, Liu JHK, Weinreb RN. Intraocular pressure measurements throughout the 24 h. Curr Opin Ophthalmol. 2009;20(2):79-83. doi:10.1097/ICU.0b013e32831eef4f
- Vahedian Z, Salmanroghani R, Fakhraie G, et al. Pseudoexfoliation syndrome: Effect of phacoemulsification on intraocular pressure and its diurnal variation. J Curr Ophthalmol. 2015;27(1-2):12-15. doi:10.1016/j.joco.2015.09.006
Identifier: Moran_CORE_127006
Copyright: Tara Gallant and Barbara Wirostko ©2023. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
Neuropathic Ocular Pain
Home / Neuro-Ophthalmology / Headaches and Positive Visual Phenomena
Title: Neuropathic Ocular Pain
Authors: Jaxon J. Huang, 4th year medical student, University of Hawaii; Anat Galor, MD MSPH, University of Miami, Bascom Palmer Eye Institute
Date: 8/15/2023
Keywords/Main Subjects: neuropathic ocular pain, eye pain, headache, photophobia, dry eye
Diagnosis: Neuropathic ocular pain
Description of Case:
A 49-year-old female with a history of depression, fibromyalgia, and traumatic brain injury presented to the ophthalmology clinic for dry eye symptoms of irritation and grittiness in both eyes for 2 years. She also reported burning and aching eye pain, frequent headaches, and sensitivity to light and wind. The patient had been using artificial tears 5 times a day for the past 6 months with no relief in symptoms. Her dry eye and ocular pain symptoms were evaluated using questionnaires, including the 5 Item Dry Eye Questionnaire (DEQ5; scale 0-22)1, Ocular Surface Disease Index (OSDI; scale 0-100)2, and the Neuropathic Pain Symptom Inventory modified for the Eye (NPSI-Eye; scale 0-40)3. Her baseline scores were as follows: DEQ5=16 (severe), OSDI=87.5 (severe), NPSI-Eye=27 (severe).
Examination:
- InflammaDry tear test (Quidel, San Diego)4: negative OD; negative OS
- Upper eyelid laxity determined by rotation (0=0-25%; 1=25-50%; 2=50-100%)5: 0 OD; 0 OS
- Lower eyelid laxity determined by snap back test (0=prompt snapback; 1=slowed return; 2=does not return until blinking)5: 0 OD; 0 OS
- Anterior blepharitis (0=none; 1=mild; 2=moderate; 3=severe)6: 1 OD; 1 OS
- Telangiectasias of the lower eyelids (0=none; 1=mild; 2=moderate; 3=severe)6: 0 OD; 1 OS
- Inferior meibomian gland plugging (0=none; 1=less than 1/3; 2=between 1/3 and 2/3; 3=greater than 2/3 lid involvement)6: 1 OD; 1 OS
- Tear break-up time (TBUT)7: 8 OD; 9 OS
- Conjunctivochalasis assessed nasally, medially, and temporally (0=none; 1=mild; 2=moderate; 3=severe)7: 1/0/1 OD; 1/0/1 OS
- Corneal staining assessed inferiorly, nasally, superiorly, temporally, and centrally then summed (0=none; 1=mild; 2=moderate; 3=severe)7: 4 OD; 3 OS
- Ocular pain before anesthesia (scale of 0-10; 0=no pain; 10=most severe pain)8: 7
- Ocular pain after anesthesia (scale of 0-10; 0=no pain; 10=most severe pain)8: 8
- Schirmer’s test (mm of wetting at 5 minutes)7: 20 OD; 9 OS
- Meibum quality (0=clear; 1=cloudy; 2=granular; 3=toothpaste; 4=no meibum extracted)6: 0 OD; 0 OS
- Inferior eyelid meibomian gland dropout graded to the Meiboscale (0=no dropout; 1=<25% dropout; 3=25% to 75% dropout; 3=>75% dropout)9: 1 OD; 1 OS
Imaging:
- In-vivo confocal microscopy10: 5+ activated dendritic cells, increased corneal nerve tortuosity, decreased corneal nerve density
Images:

Figure 1. In-vivo confocal microscopy image of corneal nerves showing activated dendritic cells (white dashed circles) and increased nerve tortuosity with decreased nerve density (white arrow).
Clinical Course:
The patient was diagnosed with neuropathic ocular pain. She was treated with 35 units of Botulinum toxin A (BoNT-A) based on a modified migraine protocol targeting the corrugator, procerus, and the frontalis muscles.11 To target photophobia, the patient was prescribed FL-41 tinted lenses. While she still reported occasional symptoms of ocular irritation, the frequency and severity of her ocular pain and migraines were reduced. Additionally, she endorsed a reduction in photophobia with use of the FL-41 tinted lenses. Her post-treatment questionnaire scores evaluating dry eye and ocular pain symptoms were as follows: DEQ5=10 (mild-moderate), OSDI=79 (severe), NPSI-Eye=17 (moderate).
Discussion:
Ocular pain is frequently incorporated under the term “dry eye disease (DED)”, which encompasses a variety of symptoms such as dryness, irritation, and pain, along with clinical signs of decreased tear production or loss of tear film homeostasis. DED pain symptoms in particular can be driven by nociceptive sources, such as ocular surface inflammation and tear film instability, or by neuropathic sources, such as nerve dysfunction.10, 12, 13 Currently, a diagnosis of a neuropathic contributor to ocular pain is made based on clinical examination findings. Individuals with neuropathic ocular pain (NOP) often describe their pain as “burning” or “shooting” and endorse evoked pain to triggers such as wind and light.14 Additionally, individuals will often present with a discordance between pain symptoms and clinical signs of tear dysfunction, with symptoms that are out of proportion to signs.15 Furthermore, persistent pain after application of a topical anesthetic and abnormal corneal nerve anatomy and function may be observed in NOP.8,16 Individuals with NOP often fail treatments targeted towards improving tear health (e.g. artificial tears and anti-inflammatory drops) and have co-morbid pain conditions, including migraine and fibromyalgia.17, 18
The current treatment of NOP involves targeting underlying nerve dysfunction using topical, oral, or adjuvant therapy. Topical therapies include autologous serum tears and transient receptor potential vanilloid (TRPV1) antagonists19, while oral agents include gabapentin, pregabalin, and nortriptyline, all of which have varying success in treating NOP.20,21 Adjuvant therapies consist of transcutaneous electrical nerve stimulation devices and BoNT-A injections, which have both been shown to decrease symptoms of ocular pain and photophobia.22, 23 In addition, the use of FL-41 tinted lenses has been reported to be helpful in alleviating photophobia and migraine.24 However, despite the wide variety of treatment options available, pain persists in many NOP patients, highlighting the need for new therapeutic approaches.25
Summary of the Case:
NOP is diagnosed clinically based on findings of “burning” pain, pain evoked by wind or light, symptoms that outweigh clinical signs of tear dysfunction, persistent pain after topical anesthetic, and abnormal corneal anatomy and function. While various topical, oral, and adjuvant therapies have been investigated and shown to have varying efficacy, further studies are needed to develop novel approaches for patients who do not respond to current options.
References:
- Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye 2010;33(2):55-60.
- Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;118(5):615-21.
- Farhangi M, Feuer W, Galor A, et al. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain 2019;160(7):1541-50.
- Sambursky R, Davitt WF, 3rd, Latkany R, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol 2013;131(1):24-8.
- Ansari Z, Singh R, Alabiad C, Galor A. Prevalence, risk factors, and morbidity of eye lid laxity in a veteran population. Cornea 2015;34(1):32-6.
- Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf 2003;1(3):107-26.
- Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5(2):108-52.
- Crane AM, Feuer W, Felix ER, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol 2017;101(9):1238-43.
- Pult H, Riede-Pult B. Comparison of subjective grading and objective assessment in meibography. Cont Lens Anterior Eye 2013;36(1):22-7.
- Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf 2017;15(3):404-37.
- Venkateswaran N, Hwang J, Rong AJ, et al. Periorbital botulinum toxin A improves photophobia and sensations of dryness in patients without migraine: Case series of four patients. Am J Ophthalmol Case Rep 2020;19:100809.
- Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf 2017;15(3):438-510.
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf 2017;15(3):276-83.
- Kalangara JP, Galor A, Levitt RC, et al. Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens 2017;43(3):192-8.
- Ong ES, Felix ER, Levitt RC, et al. Epidemiology of discordance between symptoms and signs of dry eye. Br J Ophthalmol 2018;102(5):674-9.
- Galor A, Felix ER, Feuer W, et al. Corneal Nerve Pathway Function in Individuals with Dry Eye Symptoms. Ophthalmology 2021;128(4):619-21.
- Galor A, Batawi H, Felix ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol 2016;100(6):745-9.
- Lee Y, Kim M, Galor A. Beyond dry eye: how co-morbidities influence disease phenotype in dry eye disease. Clin Exp Optom 2022;105(2):177-85.
- Patel S, Mittal R, Sarantopoulos KD, Galor A. Neuropathic ocular surface pain: Emerging drug targets and therapeutic implications. Expert Opin Ther Targets 2022;26(8):681-95.
- Small LR, Galor A, Felix ER, et al. Oral Gabapentinoids and Nerve Blocks for the Treatment of Chronic Ocular Pain. Eye Contact Lens 2020;46(3):174-81.
- Ozmen MC, Dieckmann G, Cox SM, et al. Efficacy and tolerability of nortriptyline in the management of neuropathic corneal pain. Ocul Surf 2020;18(4):814-20.
- Sivanesan E, Levitt RC, Sarantopoulos CD, et al. Noninvasive Electrical Stimulation for the Treatment of Chronic Ocular Pain and Photophobia. Neuromodulation 2018;21(8):727-34.
- Reyes N, Huang JJ, Choudhury A, et al. Botulinum toxin A decreases neural activity in pain-related brain regions in individuals with chronic ocular pain and photophobia. Front Neurosci 2023;17:1202341.
- Katz BJ, Digre KB. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol 2016;61(4):466-77.
- Siedlecki AN, Smith SD, Siedlecki AR, et al. Ocular pain response to treatment in dry eye patients. Ocul Surf 2020;18(2):305-11.
Faculty Approval by: Griffin Jardine, MD
Identifier: Moran_CORE_126997
Copyright: Jaxon J. Huang and Anat Galor ©2023. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
Case Report and Clinical Features of Elschnig Pearls
Home / Lens and Cataract / Complications of Cataract Surgery
Title: Case Report and Clinical Features of Elschnig Pearls
Authors: Marissa Larochelle, MD; Wyatt Corbin, BS
Date: 9/22/2023
Keywords/Main Subjects: Elschnig pearl, cataract surgery, PCO, posterior capsular opacification
Diagnosis: Elschnig Pearls
Images or video:

Image 1. Slit-lamp exam of the patient’s left eye’s anterior surface. Cloudy material consisting of Elschnig pearls is protruding from the superonasal and inferotemporal quadrants and obscuring the visual axis. Some of the material may also be seen extruding anteriorly from behind the inferotemporal iris at the 5 o’clock position. The iris is poorly dilated and moderate iris bombe is also present.

Image 2. Magnified image of a large amount of round, individually translucent yet collectively opaque Elschnig pearls protruding from the superonasal and inferotemporal quadrants and obscuring the visual axis; PCIOL is present and appears centered. Individual Elschnig pearls may be visualized in this image.

Image 3. Slit-lamp exam of the patient’s left eye’s anterior surface after surgical peeling and aspiration of the Elschnig pearls. Unlike the comparative preoperative photo, no Elschnig pearls are seen in this image and the visual axis appears clear.
Case Report
Patient medical history:
A 67-year-old female was referred for the surgical treatment of Elschnig pearls. A uveitis colleague noted decreased visual acuity due to Elschnig pearls on slit lamp exam one month prior. The patient described having “filmy vision” in her left eye with light sensitivity. The visual acuity in the left eye was 20/40 with no improvement with pinhole testing, a decrease from her baseline vision of 20/20 documented 3 months prior. Ultrasound biomicroscopy confirmed the presence of material on the anterior surface of the IOL optic consistent with our clinical diagnosis.
The patient has an ocular history of pseudophakia of both eyes, non-granulomatous anterior uveitis in both eyes, and intermediate uveitis in her left eye. Cataract surgery was performed nearly 17 years prior in the left (affected eye). For her ocular inflammatory conditions, she was taking Prednisolone BID OU, methotrexate 25mg weekly, and folic acid 1mg daily. The patient also has a history of type 2 diabetes mellitus, hypothyroidism, hypertension, chronic sinusitis, cervicalgia, and headache.
Examination:
The patient presented with a visual acuity of 20/20 in the right eye and a visual acuity of 20/40 with no improvement with pinhole testing in the left eye. At the current visit, she had a normal eye pressure reading of 10 in the left eye. The patient did not have any afferent pupillary defects, although the left eye had minimal reactivity to light. All extraocular movements were intact.
The left eye presented with extensive inferotemporal and superonasal Elschnig pearls anterior and posterior to the PCIOL and obscuring the visual axis (see Images 1 and 2). The Elschnig pearls were also seen extruding anteriorly from behind the inferotemporal iris at the 5 o’clock position (see Image 1). The left eye also had old pigmented keratic precipitates inferiorly, a quiet anterior chamber and a centered posterior chamber intraocular lens. Otherwise, the external and fundus exams were normal.
Management:
Due to the extensive amount of Elschnig pearls in the visual axis, the patient was managed with anterior chamber washout via surgical peeling and aspiration (see Image 3). This was done in the operating room with bimanual irrigation and aspiration. She was treated prophylactically with Prednisolone acetate 1% drops four times daily starting 1 week before the surgery to prevent a uveitis flare in the peri-operative period.
After a successful surgery, the patient’s visual axis became clear. However, her visual acuity did not improve. We suspect that the patient’s lack of visual improvement post-operatively may be due to their long-standing history of uveitis.
Clinical Features of Elschnig Pearls
Definition and Background Information: Elschnig pearls are cystic, giant cell-like structures that are thought to be a manifestation of regenerative posterior capsular opacification (PCO) caused by residual equatorial lens epithelial cell (LEC) migration and proliferation between the posterior capsule and the intraocular lens (IOL) after cataract surgery.1 The exact morphology and histology of Elschnig pearls are yet to be fully elucidated, but they have been described as containing a nucleus and few cell organelles.1,2 Elschnig pearls are also thought to be products of the ballooning of cytoplasm emerging from the cell membrane of degenerating lens fibers.1,3 Interestingly, another case report of Elschnig pearls in a patient with chronic uveitis was published, suggesting a possible mechanism of development associated with intraocular inflammation.4 However, one study reported that individual variability may have a greater effect on their formation than the degree of inflammation.4,5
The epidemiology of Elschnig pearls is unknown because they have a variable presentation and thus are difficult to distinguish from other forms of PCO.4 For example, our patient presented with debris, which was revealed to be Elschnig pearls, posterior and anterior to the IOL optic, rather than the more common clinical presentation of PCO posterior to IOL optics. Also, although they were once considered rare, they are now regarded as a relatively common post-operation complication of cataract surgery.4,6 It is not known exactly why they are regarded as a more common complication now. However, it may be due to enhanced diagnostic techniques including ultrasound biomicroscopy increasing their detection rate, or another factor related to the current population or modern cataract surgery practices resulting in increased Elschnig pearl formation. More research would be needed to elucidate why their prevalence has seemed to increase.
Diagnosis: Like other forms of PCO, Elschnig pearls are diagnosed in clinical settings via slit-lamp microscopy. They appear as cloudy clusters of pearls, most commonly posterior to PCIOLs and anterior to the posterior lens capsule. However, they may also present anterior to PCIOLs yet mostly contained within the posterior chamber such as in our patient.
Symptoms: Like other forms of PCO, Elschnig pearls do not always cause symptoms, but they can lead to a decrease in visual acuity and contrast sensitivity due to light scattering. It is proposed that the cellular material inside the Elschnig pearls has a higher refractive index which results in this light scattering.1,4,7-9
Prognosis: The presence of Elschnig pearls generally does not resolve spontaneously, although there are case reports of rare spontaneous regression.10,11 In part, spontaneous regression is rare because when visual symptoms occur the pearls are often treated immediately by neodymium yttrium garnet (Nd:YAG) laser capsulotomy. Like other forms of PCO, however, Elschnig pearls may continually progress or remain stable over time.1,4,12 Regression, with or without the assistance of laser or surgical intervention, generally occurs through pearls falling through a capsulotomy posteriorly into the vitreous, phagocytosis of the pearls by macrophages, or apoptotic cell death.6,12 Although the presence of Elschnig pearls in a patient is generally persistent for months to years, one study demonstrated that individual pearls may appear and disappear within days, most likely via different mechanisms as stated earlier.1 The progression and regression of individual pearls were observed to be influenced by the size, shape, and solidity of the pearls, as well as variability between patients.1,4,5
Management: Similar to other forms of PCO, Elschnig pearls may only need to be treated if visual symptoms occur, thus patient monitoring with patient reports, functional vision testing, and slit-lamp examination is a part of management. For example, although one case report presented a patient with Elschnig pearls obstructing his central visual axis, his vision was still 20/15, so no treatment was performed.10 When symptomatic, the primary treatment option for the pearl or regeneratory form of PCO is Nd:YAG laser capsulotomy due to the convenience of the procedure and decreased risks associated with surgical treatment. Additionally, an anterior chamber washout by surgical peeling, irrigation, and aspiration may be performed.13 Notably, the fibrous form of PCO is only treated by Nd:YAG laser capsulotomy.13 Patients with other complications secondary or unrelated to the Elschnig pearls, prior cataract surgery, or potential underlying causes of inflammation, such as uveitides causing synechiae, must also be managed via the appropriate surgical or medical interventions. Chronic medically induced pupil dilation to allow for a greater visual window and bimanual anterior vitrectomy may also be considered as treatment options for patients with Elschnig pearls.
One study compared Nd:YAG laser capsulotomy with surgical peeling and aspiration and showed that both techniques are comparable with regard to visual outcomes.13 However, Nd:YAG laser capsulotomy was associated with a higher incidence of spikes in intraocular pressure (IOP) and retinal detachment whereas treatment with surgical peeling and aspiration was associated with a higher incidence of pearl recurrence. Thus, caution must be exercised when considering Nd:YAG laser capsulotomy in patients with previous retinal disease and pathologic myopia.13
After Nd:YAG laser capsulotomy or surgical peeling, irrigation, and aspiration, the patient must be monitored to diagnose and treat any post-operative inflammation or spikes in IOP. Repeat treatment may also be necessary to treat recurring Elschnig pearls by utilizing any of the aforementioned methods based on clinical judgment.
Summary of the Case:
We present a case and two images of Elschnig pearls in a 67-year-old female patient with a history of pseudophakia in her ipsilateral eye and anterior segment uveitis. Given this patient’s clinical background and the extent of growth of Elschnig pearls, an anterior chamber washout with surgical peeling, irrigation, and aspiration was performed successfully.
Elschnig pearls are a relatively common regenerative form of PCO after cataract surgery and are currently thought to be cystic, cell-like structures that are products of lens fiber cells that degenerate after cataract surgery. The most common risk factor for Elschnig pearls is previous cataract surgery, although chronic inflammation and other patient-specific factors may present varying risks for pearl formation. Patients with Elschnig pearls may experience visual disturbances such as photopsias which are commonly persistent. They are primarily treated with Nd:YAG laser capsulotomy. Although if clinically appropriate, they may also be treated surgically with an anterior chamber washout via irrigation and aspiration. Each treatment modality is accompanied by risks and benefits that must be weighed by each clinician when considering the safest and most optimal treatment for specific patients.
Format: Case Report and Clinical Features
References:
-
- Findl O, Neumayer T, Hirnschall N, Buehl W. Natural Course of Elschnig Pearl Formation and Disappearance. Investigative Opthalmology & Visual Science. 2010;51(3):1547. doi:10.1167/iovs.09-3989
- COWAN A, FRY. SECONDARY CATARACT. Archives of Ophthalmology. 1937;18(1):12. doi:10.1001/archopht.1937.00850070024002
- Jongebloed WL, Kalicharan D, Los LI, van der Veen G, Worst JG. A combined scanning and transmission electronmicroscopic investigation of human (secondary) cataract material. Doc Ophthalmol. 1991;78(3-4):325-334. doi:10.1007/BF00165696
- K Foutch B, A Garcia C, S Ferguson A. Pearls of Elschnig. J Ophthalmic Vis Res. 2019;14(4):525-527. doi:10.18502/jovr.v14i4.5469
- Neumayer T, Buehl W, Findl O. Effect of topical prednisolone and diclofenac on the short-term change in morphology of posterior capsular opacification. Am J Ophthalmol. 2006;142(4):550-556. doi:10.1016/j.ajo.2006.04.047
- Caballero A, Salinas M, Marin JM. Spontaneous disappearance of Elschnig pearls after neodymium:YAG laser posterior capsulotomy. J Cataract Refract Surg. 1997;23(10):1590-1594. doi:10.1016/s0886-3350(97)80035-1
- Brown N. Visibility of transparent objects in the eye by retroillumination. Br J Ophthalmol. 1971;55(8):517-524. doi:10.1136/bjo.55.8.517
- Buehl W, Sacu S, Findl O. Association between intensity of posterior capsule opacification and contrast sensitivity. Am J Ophthalmol. 2005;140(5):927-930. doi:10.1016/j.ajo.2005.05.022
- Jose RMJ, Bender LE, Boyce JF, Heatley C. Correlation between the measurement of posterior capsule opacification severity and visual function testing. J Cataract Refract Surg. 2005;31(3):534-542. doi:10.1016/j.jcrs.2004.07.022
- Nakashima Y, Yoshitomi F, Oshika T. Regression of Elschnig pearls on the posterior capsule in a pseudophakic eye. Arch Ophthalmol. 2002;120(3):397-398.
- Caballero A, Marín JM, Salinas M. Spontaneous regression of Elschnig pearl posterior capsule opacification. J Cataract Refract Surg. 2000;26(5):779-780. doi:10.1016/s0886-3350(00)00412-0
- Kurosaka D, Kato K, Kurosaka H, Yoshino M, Nakamura K, Negishi K. Elschnig pearl formation along the neodymium:YAG laser posterior capsulotomy margin. Long-term follow-up. J Cataract Refract Surg. 2002;28(10):1809-1813. doi:10.1016/s0886-3350(02)01222-1
- Bhargava R, Kumar P, Sharma SK, Kaur A. A randomized controlled trial of peeling and aspiration of Elschnig pearls and neodymium: yttrium-aluminium-garnet laser capsulotomy. Int J Ophthalmol. 2015;8(3):590-596. doi:10.3980/j.issn.2222-3959.2015.03.28
Faculty Approval by: Austin Nakatsuka, MD
Copyright statement: Marissa Larochelle and Wyatt Corbin ©2023. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
A Brief Overview of Salzmann’s Nodular Degeneration and Its Topographical Outcomes
Home / External Disease and Cornea / Clinical Approach to Depositions and Degenerations of the Conjunctiva, Cornea, and Sclera
Author: Tanmay Majmudar, BS, MS4 at Drexel University College of Medicine
Photographer: James Gilman, CRA FOPS
Date: 9/12/2023
Keywords/Main Subject: cornea, corneal dystrophy, corneal topography, Salmann’s nodular degeneration, superficial keratectomy
Diagnosis: Salzmann’s Nodular Degeneration
Case Presentation:
A 64-year-old female was referred to the cornea clinic by an outside optometrist for progressively worsening vision in both eyes (OS worse than OD). She had a remote history of pterygium removal in both eyes, high astigmatism and hyperopia corrected with bifocal prescription glasses, and early dry stage nonexudative age-related macular degeneration. She also used preservative free artificial tears twice daily for chronic eye dryness and bedtime lubricant ointment as needed. Her past medical, family, and social histories were otherwise unremarkable.
Upon presentation to our clinic, her best corrected visual acuity (BCVA) with prescription bifocals was 20/40-1 and 20/60 in the right and left eyes respectively. Slit lamp examination of the anterior segment revealed a 2.5 mm greyish nodule (see a representative slit-lamp image from another patient with the same diagnosis in Figure 1) between the corneal epithelium and Bowman’s layer nasally over the pterygium scar in the right eye and a similar appearing 4 mm nodule nasally in the left eye as well. Trace to 1+ superficial punctate keratitis stable from prior exams was also noted in both eyes. The rest of the exam, including a dilated fundus exam, was unremarkable besides nuclear sclerotic cataracts (graded 2+) in both eyes.

Figure 1. Slit-lamp image of another patient with Salzmann’s nodular degeneration showing the characteristic blue-greyish nodules. Image taken by James Gilman, University of Utah John A Moran Eye Center.
Corneal Topography:
Atlas corneal topography was ordered to characterize the corneal nodules seen on slit-lamp examination, especially given the clinical history of astigmatism. Figure 2 shows a Zeiss Atlas report of the patient for both eyes with an axial curvature map and Placido disc-based “rings image”. Topography in the left eye shows a high irregular astigmatism with focal steepening and irregular mires noted in the superotemporal region. A crab-claw like pattern of irregular astigmatism is more noticeable nasally in the right eye. These focal areas of astigmatism largely correspond to the location of the blue-grayish nodules noted on slit-lamp examination.
Diagnosis:
A diagnosis of Salzmann’s nodular degeneration (SND) was made on the basis of the characteristic appearance on slit-lamp examination, clinical history of bilateral, gradually progressive, painless visual decline, history of prior ocular surface surgery (pterygium removal) and chronic dry eyes, and high irregular astigmatism noted on corneal topography corresponding to areas of nodular growth.
Treatment & Clinical Course:
To improve visual acuity and to reduce the high irregular astigmatism, a superficial keratectomy (SK) was recommended to remove the Salzmann’s nodules; however, the patient was informed that refractive outcomes may not eliminate her astigmatism and that recurrence of these nodules was possible.
Post-Operative Outcomes:
About two weeks after her initial presentation to the cornea clinic, the patient underwent SK on her left eye. At the time of writing, post-operative refractive and topographical data for the patient’s right eye (surgery completed about two weeks after the left eye) are unavailable. Two months postoperatively, her left cornea’ epithelium healed with complete clearing of the subepithelial opacities. Post-operatively her left eye achieved a best corrected visual acuity of 20/20 -2, with substantial reduction in astigmatic error (Figure 3, Table 1).
Table 1. Pre- and post-operative refraction in a patient with Salzmann’s nodular degeneration.
| Pre-operative Manifest Refraction (OS) | |
| -0.25 + 1.50 x 045 | VA 20/60 |
| Post-operative Manifest Refraction | |
| -0.50 + 1.00 x 030 | VA 20/20 -2 |

Figure 3. Left eye topography before (top row) and after (bottom row) superficial keratectomy for removal of Salzmann’s nodules. After intervention, topography shows a more spherical cornea with substantially more regular mires and corresponding reduction in both regular and irregular astigmatism from 5.36 diopters to 1.00 D.
Discussion:
Salzmann’s Nodular Degeneration (SND) is an unusual and relatively rare1 disorder of the cornea that involves the growth of blue-grayish fibrous corneal opacities of various sizes (typically 1-3 mm) anterior to the Bowman’s capsule2. It is a slowly progressive, noninflammatory degenerative condition that typically affects middle-aged Caucasian females (between ages 50-60)2,3 and occurs bilaterally3. Though many patients with SND are asymptomatic early in their disease course, gradually decreasing visual acuity due to irregular astigmatism, hyperopic shifts, or mechanical tear film disturbances is the most common presenting symptom3-5. Other symptoms are related ocular surface irritation, including foreign body sensation, pain, tearing, blepharospasms, or recurrent corneal erosions are also reported2,3,6.
The precise mechanism of this degeneration is poorly understood. Mechanical disruption between the corneal epithelium and stroma from chronic ocular surface inflammatory conditions such as dry eye, meibomian gland dysfunction (MGD), long-term contact lens wear, keratoconjunctivitis sicca, exposure keratitis, pterygium amongst others have been implicated in prior studies2,3,6-9. SND has also been observed in the setting of previous ocular surgery and trauma, such as after LASIK5,10, cataract extraction11, radial keratotomy12, and corneal transplants13. Our patient’s history of chronic eye disease, pterygium removal, and long-term contact lens wear also supports the pathogenic role of these risk factors in the development of SND. Finally, prior studies have documented associations between systemic inflammatory conditions, such as Crohn’s disease14, and SND while others have also attempted to elucidate a genetic involvement15.
Diagnosis of SND is usually made based on a careful examination of corneal structure under slit-lamp biomicroscopy. Nodules appear as blueish-white to grayish-yellow subepithelial elevations that may occasionally stain with fluorescein2. While diagnosis of SND is primarily based on slit-lamp examination findings and the clinical history, it may be supported by corneal topography, as in this patient, or high-frequency ultrasound biomicroscopy, in-vivo confocal microscopy, and optical coherence tomography of the anterior segment. Classic histologic features include a thinned epithelium overlying the nodules, fragmented or absent Bowman’s membrane, or stromal scarring2,16. Unfortunately, these histologic features of non-specific and can frequently be seen with old corneal scars from trauma or inflammation.
Management options are largely driven by the severity of symptoms. Although asymptomatic patients are primarily observed, treatment of mild disease is aimed at addressing the underlying etiology through medical management. Use of preservative-free lubricant eye drops, lid hygiene, and warm compresses in patients with chronic dry eye has been effective in the majority of cases3. Medical options to treat SND associated with chronic ocular inflammation include steroids, NSAIDs, cyclosporine and doxycycline amongst others2. If nodules occur in the setting of contact lens use, then reduction of use or cessation may also relieve symptoms. However, in cases that do not respond to conservative management or those patients with visually significant nodules, surgical management is necessary.
A variety of surgical options exist, including SK, otherwise known as Salzmann nodulectomy, phototherapeutic keratectomy (PTK), lamellar or penetrating keratoplasty. Of these, SK is the most common method for nodule removal. Briefly, the epithelium overlying the nodules is removed and the nodular edge is simply peeled off the corneal surface with forceps or a flat blade, leaving Bowman’s layer almost untouched2. Post-operative outcomes are very favorable in reducing astigmatic error and improvements in visual acuity have been recorded in 79.2%-100% of patients in prior studies2,3,6. In rare cases, persistent anterior stromal haze may necessitate further treatment with PTK. Rarely, lamellar or penetrating keratoplasty may be required for patients with concurrent corneal pathologies involving the deeper stroma and endothelium. Although varying rates of recurrences have been reported3,6,7, visually significant recurrences are uncommon (ranging from 5 to 20%) and can be further minimized with management of the underlying etiologies3,6.
Beyond the visual limitations of corneal nodularity itself, the accuracy of keratometry and biometry calculations, a critical step in selecting the appropriate intraocular lens (IOL), is largely based on a clear cornea with a smooth regular surface. Patients with corneal dystrophies, such as SND or epithelial basement membrane dystrophy, have irregular corneas that can such adversely affect biometry, recommended IOL choice, and postoperative visual outcomes. In patients requiring cataract surgery who also have comorbid corneal irregularities, prior studies have showed improved reliability in biometry measurements after management with SK and better post-cataract optical quality17,18. Although our patient, with 2+ nuclear sclerotic cataracts, continues to deny visually limiting symptoms related to them, this case nonetheless highlights the importance of addressing the refractive sequelae of SND in consideration for future cataract surgery.
References:
- Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. Sep 2018;44(9):1090-1096.
- Hamada S, Darrad K, McDonnell PJ. Salzmann’s nodular corneal degeneration (SNCD): clinical findings, risk factors, prognosis and the role of previous contact lens wear. Cont Lens Anterior Eye. Aug 2011;34(4):173-8.
- Graue-Hernández EO, Mannis MJ, Eliasieh K, et al. Salzmann nodular degeneration. Cornea. Mar 2010;29(3):283-9.
- Oster JG, Steinert RF, Hogan RN. Reduction of hyperopia associated with manual excision of Salzmann’s nodular degeneration. J Refract Surg. Jul-Aug 2001;17(4):466-9.
- VanderBeek BL, Silverman RH, Starr CE. Bilateral Salzmann-like nodular corneal degeneration after laser in situ keratomileusis imaged with anterior segment optical coherence tomography and high-frequency ultrasound biomicroscopy. J Cataract Refract Surg. Apr 2009;35(4):785-7.
- Farjo AA, Halperin GI, Syed N, Sutphin JE, Wagoner MD. Salzmann’s nodular corneal degeneration clinical characteristics and surgical outcomes. Cornea. Jan 2006;25(1):11-5.
- Das S, Langenbucher A, Pogorelov P, Link B, Seitz B. Long-term outcome of excimer laser phototherapeutic keratectomy for treatment of Salzmann’s nodular degeneration. J Cataract Refract Surg. Jul 2005;31(7):1386-91.
- Vannas A, Hogan MJ, Wood I. Salzmann’s nodular degeneration of the cornea. Am J Ophthalmol. Feb 1975;79(2):211-9.
- Yoon KC, Park YG. Recurrent Salzmann’s nodular degeneration. Jpn J Ophthalmol. Jul-Aug 2003;47(4):401-4.
- He X, Donaldson KE. Superficial keratectomy for Salzmann nodular degeneration following laser in situ keratomileusis. Can J Ophthalmol. Jun 2019;54(3):e149-e151.
- Qiu J, Cai R, Zhang C. Association between poor wound healing and the formation of Salzmann nodules. J Cataract Refract Surg. Oct 2016;42(10):1527-1530.
- Fong YC, Chuck RS, Stark WJ, McDonnell PJ. Phototherapeutic keratectomy for superficial corneal fibrosis after radial keratotomy. J Cataract Refract Surg. Apr 2000;26(4):616-9.
- Geggel HS. Effect of peripheral subepithelial fibrosis on corneal transplant topography. J Cataract Refract Surg. Jan-Feb 1996;22(1):135-8.
- Roszkowska AM, Spinella R, Aragona P. Recurrence of Salzmann nodular degeneration of the cornea in a Crohn’s disease patient. Int Ophthalmol. Apr 2013;33(2):185-7.
- Papanikolaou T, Goel S, Jayamanne DG, Mudhar H, Desai SP. Familial pattern of Salzmann-type nodular corneal degeneration–a four generation series. Br J Ophthalmol. Nov 2010;94(11):1543.
- Stone DU, Astley RA, Shaver RP, Chodosh J. Histopathology of Salzmann nodular corneal degeneration. Cornea. Feb 2008;27(2):148-51.
- Goerlitz-Jessen MF, Gupta PK, Kim T. Impact of epithelial basement membrane dystrophy and Salzmann nodular degeneration on biometry measurements. J Cataract Refract Surg. Aug 2019;45(8):1119-1123.
- Kim BZ, Wilson PJ, McGhee CN. Annular Salzmann degeneration: Avoiding perturbations and pitfalls in phacoemulsification surgery. J Cataract Refract Surg. Nov 2015;41(11):2580-3.
Faculty Approval By: Brian Zaugg, MD & Griffin Jardine, MD
Copyright: Tanmay Majmudar, ©2023. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
Coats Disease Masquerading as Panuveitis
Home / Retina and Vitreous / Other Retinal Vascular Diseases
Title: Coats Disease Masquerading as Panuveitis
Authors: Christopher Le, MSIV University of Colorado School of Medicine; Eric Hansen, MD.
Date: 7/8/2023
Keywords/Main Subjects: coats disease, uveitis masquerade syndromes
Diagnosis: Coats Disease
Description of Case:
Introduction:
Coats disease is a rare, congenital, nonhereditary, idiopathic disorder of the retinal vasculature leading to massive subretinal and intraretinal exudation. The disease typically affects young males in the first or second decades of life and is unilateral.1 The pathophysiology of Coats disease remains unclear – however, it has been proposed that defects in endothelial cells of the retinal vasculature along with abnormal pericytes contribute to the breakdown of the blood-retinal barrier leading to the development of telangiectatic vessels, retinal ischemia, and exudation of lipid-rich fluid.2 Patients with Coats disease have varying symptoms on initial presentation including: decreased visual acuity, eye pain, strabismus, and leukocoria.3 Rarely, Coats disease may present with symptoms and exam findings suggestive of intraocular inflammatory conditions such as uveitis or endophthalmitis. Diseases that present with an intraocular inflammatory picture but are not caused by immune-mediated or infectious processes are referred to as uveitis masquerade syndromes (UMS).4
Case Presentation:
We present a case of Coats disease in a 14-year-old male masquerading as a panuveitis. The patient presented to the emergency department at an outside institution with a three-day history of blurred vision, pain, and redness in his left eye, as well as fever, body aches, and a facial rash. On initial examination, visual acuity in his left eye was markedly decreased at light perception only. There was conjunctival injection and diffuse cell in the anterior chamber and vitreous. On dilated funduscopic examination, a multifocal exudative retinal detachment without macular involvement was appreciated with diffuse subretinal and intraretinal yellow-white exudation and peripheral retinal hemorrhages. Interestingly, the patient reported having a normal dilated eye exam just one year prior.
Shortly after presentation to the outside facility, the patient was transferred to the Moran Eye Center at the University of Utah. Due to concern for possible endophthalmitis or infectious panuveitis, the patient underwent a vitreous and anterior chamber tap as well as injection of broad-spectrum antimicrobials. The patient was evaluated by uveitis specialist who recommended a broad uveitis workup and vitreous biopsy given a dry vitreous tap. The patient’s respiratory viral panel resulted positive for rhinovirus. Broad uveitis workup – including evaluation for HSV, VZV, CMV, toxoplasma, vitreous PCR, quantiferon, RPR, syphilis antibody, bartonella, lysozyme, ACE, and ANCA – returned unremarkable. The patient underwent an exam under anesthesia and a 27-gauge pars plana vitrectomy with vitreous biopsy and intravitreal injections of antimicrobial agents including vancomycin, ceftazidime, clindamycin, and foscarnet. Interestingly, the culture from patient’s vitreous biopsy grew pan-sensitive staphylococcus Capitis. However, the patient’s Karius panel was negative for bacteria and positive for candida. Both positive results were determined to be likely contaminants in consultation with the infectious disease department.
The patient followed-up in clinic and underwent fundus photography and fluorescein angiography following vitrectomy. Fundus examination demonstrated telangiectatic vessels and bulbs with associated diffuse subretinal exudative material and exudative retinal detachment. Fluorescein angiography highlighted the telangiectatic vessels and demonstrated terminal light bulb aneurysms, capillary nonperfusion, and perivascular leakage. The patient was diagnosed with Coats disease, stage 3A given this constellation of findings. The patient was scheduled to undergo laser photocoagulation therapy with adjuvant intravitreal therapy.
At the time of treatment, he was found to have diffuse vitreous hemorrhage obscuring a clear view of the retina with worsening exudative retinal detachment. Subsequently, he was taken to the operating room for a pars plana vitrectomy with scleral buckling, external drainage of exudative material, application of endolaser, silicone oil endotamponade, and intravitreal Avastin injection. At the time of this vitrectomy, the patient’s exudative retinal detachment had worsened to total detachment –classifying the patient’s disease as stage 3B. The patient subsequently underwent multiple exams under anesthesia with laser photocoagulation, sub-tenons Kenalog and intravitreal Avastin injections resulting in resolution of the exudative retinal detachment, significantly improved exudation, and residual fibrosis and vitreoretinal traction sparing the macula. The patient’s visual acuity has improved to counting fingers, and he remains without any signs of neovascular glaucoma.
Discussion:
This case highlights a rare and atypical presentation of Coats disease masquerading as a panuveitis. The critical step in obtaining this patient’s diagnosis, and in diagnosis of Coats disease in general, was obtaining fluorescein angiography with clear view which demonstrated pathognomonic features of the disease: telangiectatic retinal vasculature with terminal light bulb aneurysms, capillary nonperfusion, and perivascular leakage. Coats disease should be included in the differential diagnosis for young males in the first or second decades of life presenting with panuveitis and exudative retinal detachments.
Images or video:
Image 1

Image 1: Fundus photograph taken after initial vitrectomy demonstrating telangiectatic retinal vasculature, yellow subretinal exudative material, and multifocal exudative retinal detachment.
Image 2
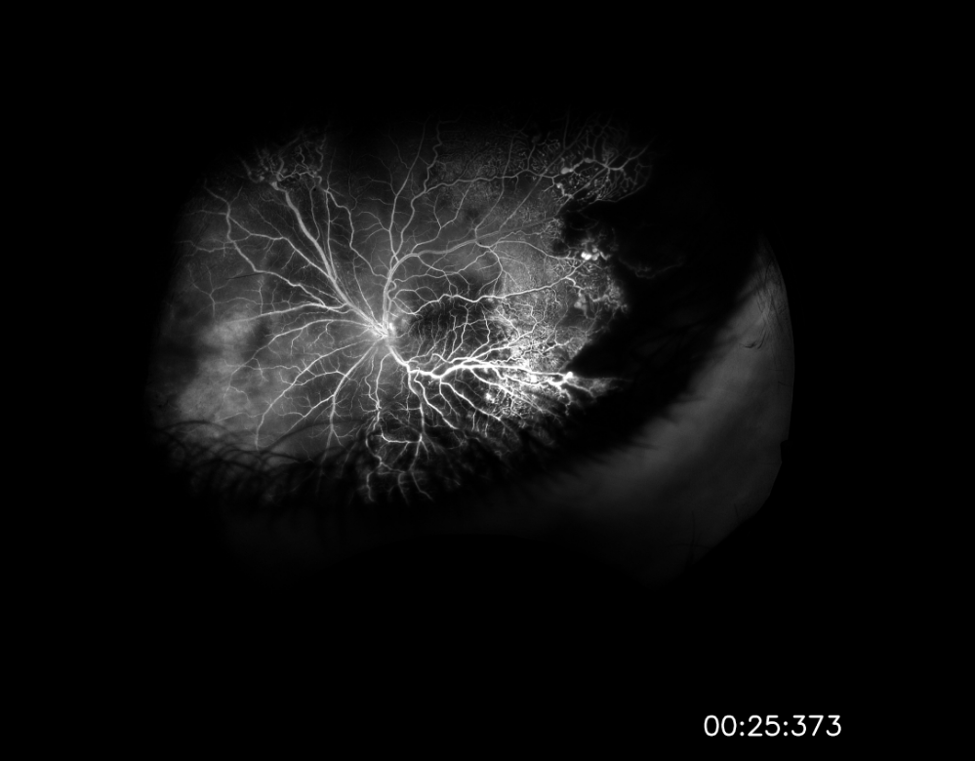
Image 2: Widefield fluorescein angiography taken after initial vitrectomy demonstrating telangiectatic vessels with terminal light bulb aneurysms, capillary nonperfusion, and perivascular leakage.
Image 3

Image 3: Fundus photograph taken during most recent exam under anesthesia in June 2023, following several laser photocoagulation and intravitreal Avastin treatments, demonstrating improved exudative retinal detachment, persistent but improved exudation, and residual fibrosis and traction involving the macula.
Summary of the Case:
A 14-year-old male presented with a marked decrease in visual acuity to light perception only, eye pain, and eye redness of the left eye in the setting of fever, chills, and a facial rash. Initial examination was remarkable for a multifocal exudative retinal detachment and significant anterior chamber and vitreous cell suggestive of a panuveitis or intraocular inflammatory condition. Vitreous culture from intraoperative biopsy grew staphylococcus Capitis, but was ultimately determined to be a contaminant. All other elements of the patient’s broad uveitis workup were unremarkable. Fluorescein angiography was performed following the patient’s initial vitrectomy and revealed telangiectatic vessels, capillary nonperfusion, perivascular leakage – findings pathognomonic for Coats disease. The patient underwent a series of interventions including vitrectomy/buckle with external drainage of exudates and several treatments with laser photocoagulation and intravitreal Avastin injections. His most recent examination and fundus photos demonstrated improvement in the exudative retinal detachment and persistent, but improved, exudation. His visual acuity has improved to counting fingers and he remains without any signs of development of neovascular glaucoma.
Format: Case Report
References
-
- Yousef YA, ElRimawi AH, Nazzal RM, et al. Coats’ disease: characteristics, management, outcome, and scleral external drainage with anterior chamber maintainer for stage 3b disease. Medicine (United States). 2020;99(16):E19623. doi:10.1097/MD.0000000000019623
- Sen M, Shields CL, Honavar SG, Shields JA. Coats disease: An overview of classification, management and outcomes. BMC Ophthalmol. 2017;17(1):1. doi:10.4103/ijo.IJO
- Yang X, Wang C, Su G. Recent advances in the diagnosis and treatment of Coats’ disease. Int Ophthalmol. 2019;39(4):957-970. doi:10.1007/s10792-019-01095-8
- Hsu YR, Wang LU, Chen FT, et al. Clinical Manifestations and Implications of Nonneoplastic Uveitis Masquerade Syndrome. Am J Ophthalmol. 2022;238(August 2020):75-85. doi:10.1016/j.ajo.2021.12.018
Faculty Approval by: Eric Hansen, MD; Griffin Jardine, MD.
Copyright: Christopher Le, © 2023. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
Identifier: Moran_CORE_126934
Enhanced S-cone Syndrome: an NR2E3-Associated Retinal Dystrophy
Home / Retina and Vitreous / Hereditary and Choroidal Dystrophies
Title: Enhanced S-cone Syndrome: an NR2E3-Associated Retinal Dystrophy
Authors: Aniket Ramshekar, PhD, MSIV, University of Utah School of Medicine and Cecinio “Nikko” Ronquillo, MD, PhD
Date: 8/23/23
Keywords/Main Subjects: enhanced s-cone syndrome, NR2E3, NRL, nyctalopia, photoreceptors
Diagnosis: Enhanced S-cone Syndrome
Description of Case: A 13-year-old male presented with impaired nighttime vision (i.e., nyctalopia) and peripheral vision loss. Past ocular history is notable for glasses since he was 4 years old. Medical and developmental history were unremarkable. Family history was unremarkable.
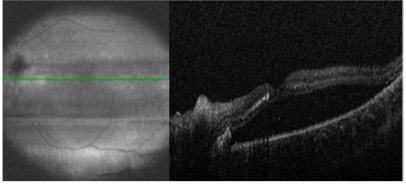
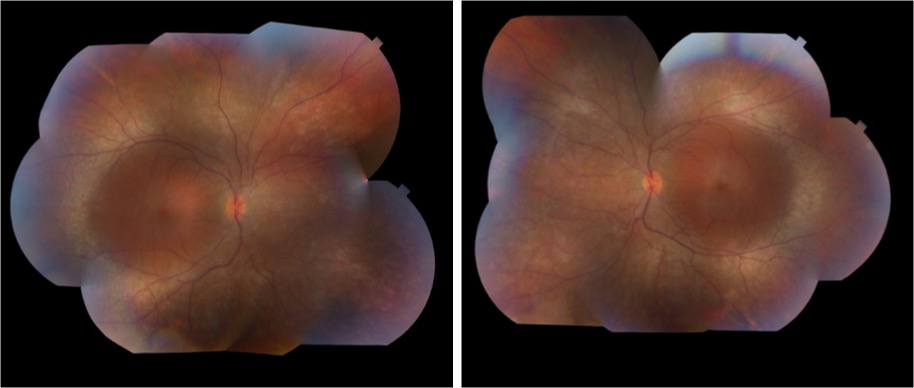
On exam, the patient had best corrected visual acuity (BCVA) of 20/30 OD and 20/40 OS, intraocular pressure (IOP) of 12 OD and 9 OS, and reduced peripheral vision to confrontation OU. Fundus exam was notable for loss of pigment, grayish appearance around the arcades and peripheral retina associated with nummular pigment changes along the superior arcade OS (Figure 1).
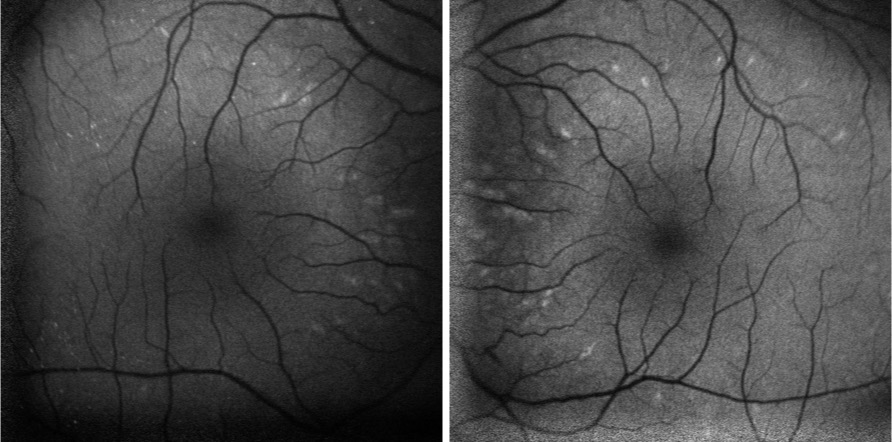
Fundus autofluorescence revealed hyper-autofluorescent spots surrounding the fovea OU (Figure 2).
Optical coherence tomography (OCT) demonstrated retained inner layers of the retina OU, cystic changes in the outer plexiform layer (OPL) OU, and loss of definition of the outer retinal layers OU (Figure 3).
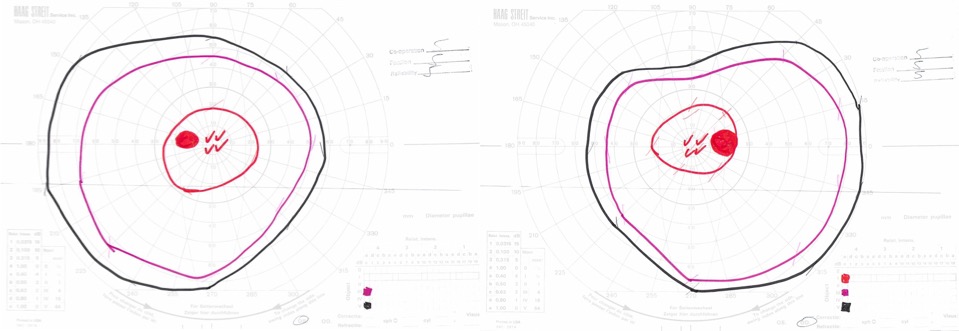
Goldmann visual field exam was full (Figure 4).
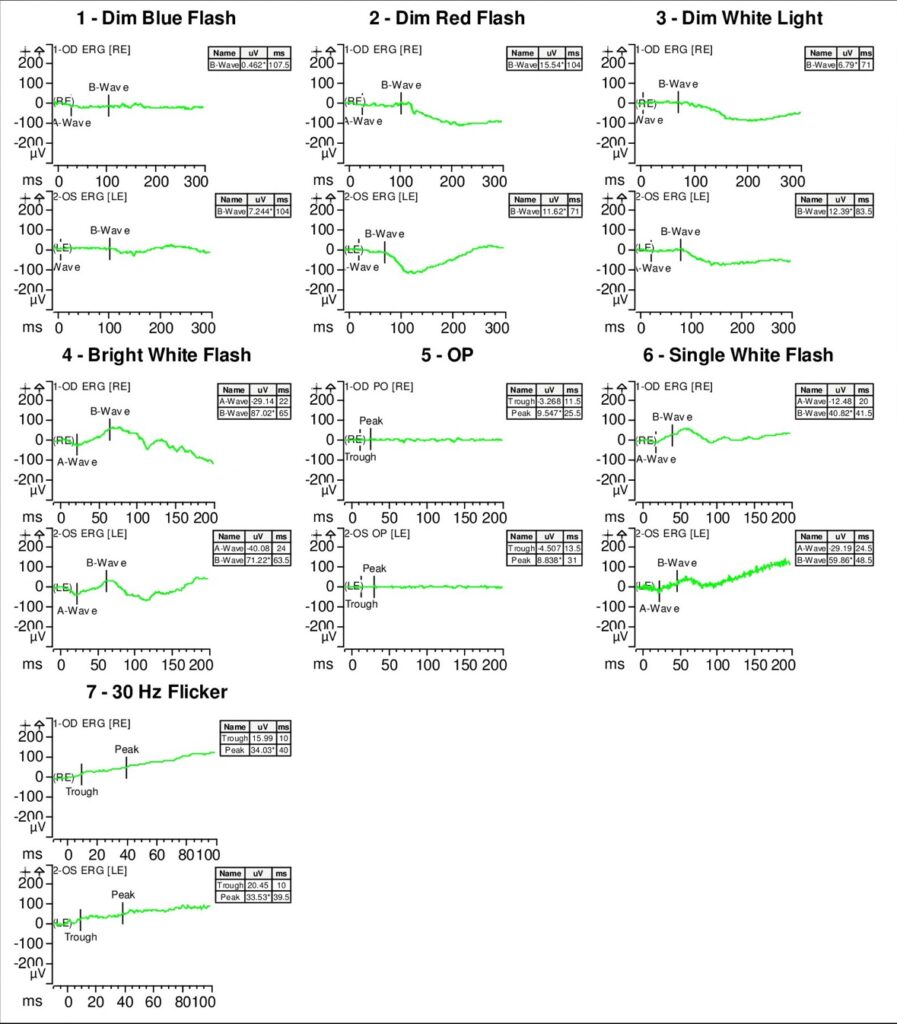
Full-field electroretinogram (ERG) showed diminished scotopic and photopic bright white flashes OU and 30 Hz flicker response that was delayed and diminished in amplitude OU (Figure 5).
Given the concern for inherited retinal dystrophy, the patient was referred for genetic testing that revealed a homozygous NR2E3 c.119-2A>C variant, which disrupts the nearby universal “AG” splice acceptor site and is likely pathogenic.
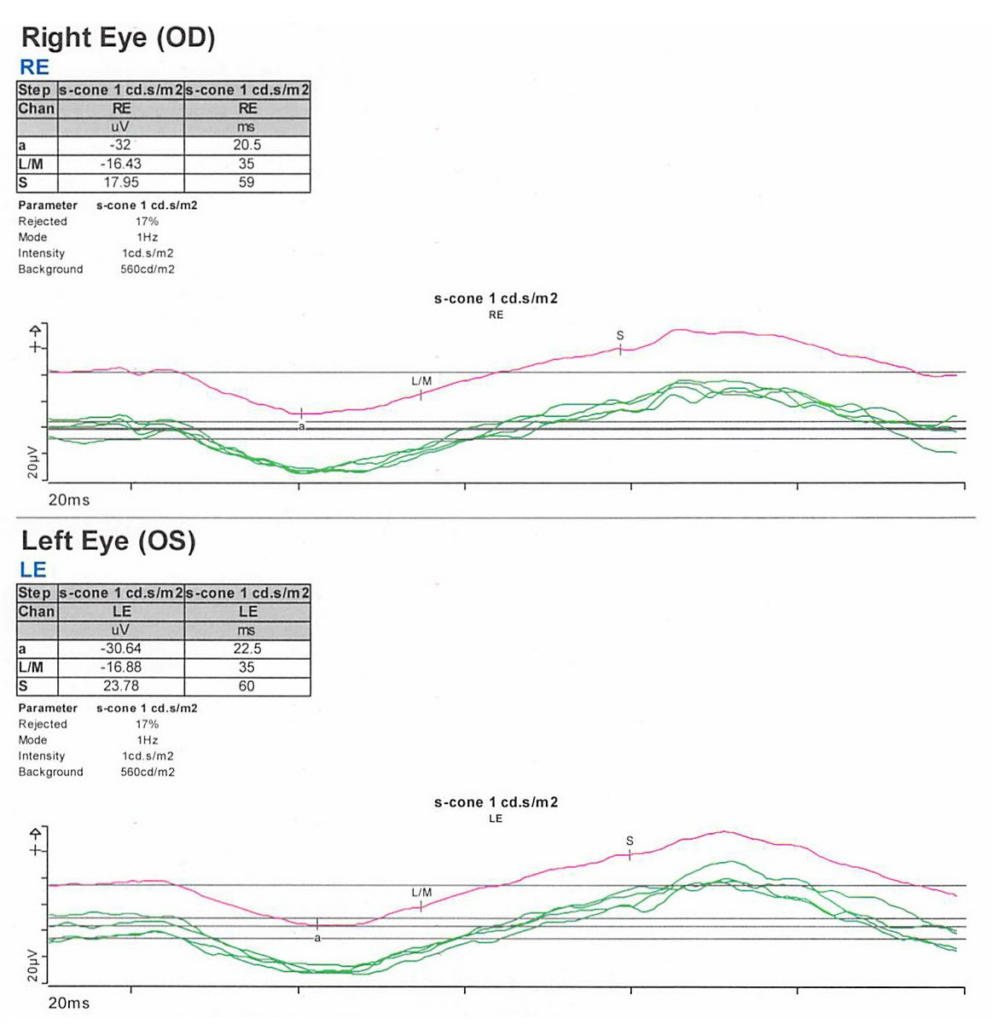
S-cone ERG, acquired in response to a blue wavelength on an orange wavelength background, demonstrated a greater than anticipated response OU (Figure 6).
Epidemiology: Enhanced S-cone syndrome (ESCS) is a rare inherited progressive retinal degeneration. The prevalence of ESCS is not well-established; however, studies have found gene mutations associated with ESCS worldwide.1-12
Genetics: ESCS is inherited in an autosomal recessive pattern. NR2E3 (Nuclear Receptor Subfamily 2, Group E, Member 3) mutations are primarily implicated in ESCS. There are over 30 pathogenic mutations in NR2E3 that have been associated with ESCS.13-15 In the United States, however, the NR2E3 c.119-2A>C variant is the most common.16
Some ESCS cases have also documented NRL (neural retina leucine zipper) mutations.17-20
Pathophysiology: NR2E3 protein is a retinal orphan nuclear receptor, a transcription factor expressed in the outer nuclear layer of the human retina.13 To gain mechanistic insights, NR2E3 mutations in mouse models have been shown to disrupt the development of rod photoreceptors and disrupt the differentiation of cone photoreceptors to L/M-cones leading to over-expansion of the S-cone population in the retina.21-23 NR2E3 expression is under the direct regulation of the transcription factor, NRL protein.24 Therefore, mutations in NRL lead to ESCS by affecting NR2E3 expression.25
Clinical presentation: The clinical presentation of ESCS is variable; however, listed below are some documented symptoms.26-28
- Night blindness (nyctalopia, due to reduced functioning rod photoreceptors)
- Increased sensitivity to blue light (due to the over-expansion of functioning S-cones)
- Reduced visual acuity
- Abnormal color vision, with a tendency to see colors as more vibrant or intense
Diagnosis: Given that the clinical presentation of ESCS is variable, the diagnostic testing might also demonstrate variable findings among patients. Below are some diagnostic findings associated with each clinical test:
- Fundus exam – Fundus exam might demonstrate torpedo-like lesions primarily located along the vascular arcades, subretinal fibrosis, nummular pigmentary changes along the vascular arcades, or intraretinal yellow dots.6,29
- ERG – full-field ERG might demonstrate a diminished rod response and similar waveform in response to both scotopic and photopic conditions. The 30 Hz flicker responses might demonstrate significantly delayed responses with attenuated amplitudes. With orange background, increased response to short wavelength (blue) might be elicited. The multifocal ERG (mfERG) might show preserved central responses, though can be delayed.6,30-35
- OCT – OCT might demonstrate cystoid macular edema (CME) or foveomacular schisis.31 There are some documented cases of subretinal fibrosis secondary to choroidal neovascularization that might also be detected by OCT.6,35-27 There was also a case of macular retinal vascularization of the posterior pole that was detected by OCT.38
- FA – In the peripheral retina, FA might demonstrate hypo-autofluorescence with patchy areas of advanced hypo-autofluorescence. In the macula, hyper-autofluorescent flecks have been documented.6 FA might also identify vascular changes (i.e., retinal or choroidal neovascularization) and complement OCT findings.
- Genetic testing can identify mutations in the NR2E3 or NRL genes and confirm the diagnosis
Differential diagnosis: Nyctalopia has a broad differential and includes conditions such as Vitamin A deficiency, cataracts, autoimmune retinopathy, glaucoma, and other inherited retinal dystrophies (i.e., congenital stationary night blindness, Oguchi’s disease, fundus albipunctatus). Therefore, a careful workup is required to narrow the differential to treat patients appropriately.
Some NR2E3 mutations are implicated in autosomal dominant retinitis pigmentosa, Goldmann-Favre syndrome, and clumped pigmentary retinal degeneration.39-41 Characterizing the underlying disease caused by the NR2E3 mutations might not improve medical management but would inform genetic counseling.
Management: There are currently no approved therapies to treat ESCS. However, CRISPR/Cas9 gene editing has been used preclinically to correct the homozygous NR2E3 c.119-2A>C splice site variant in induced pluripotent stem cells (iPSCs) from two patients with ESCS.42 This finding suggests that CRISPR/Cas9 gene editing might be a plausible treatment approach for ESCS in the future.
Current medical management includes treating foveomacular schisis and CME with carbonic anhydrase inhibitors, while choroidal neovascularization is treated with agents that inhibit vascular endothelial growth factor.43-49 Genetic testing and patient support programs should also be offered in all patient cases. Genetic penetrance and carrier frequency of the NR2E3 mutations are not well understood given the rarity of disease phenotypes. Therefore, genetic testing of the patient and family might help provide further information to improve genetic counseling.
Summary of the Case:
- ESCS is a rare inherited progressive retinal degenerative disease of the photoreceptors that can present as nyctalopia
- There is clinical and diagnostic variability among patients with ESCS, but mutations in the transcription factors, NR2E3 or NRL, are implicated in pathology
- Current treatment is limited to treating foveomacular schisis and CME with carbonic anhydrase inhibitors, and choroidal neovascularization with anti-VEGF agents
- Genetic testing should be offered to inform genetic counseling
- Studies exploring CRISPR/Cas9 gene editing show promise for future therapies
References
- Bandah, D., Merin, S., Ashhab, M., Banin, E. & Sharon, D. The spectrum of retinal diseases caused by NR2E3 mutations in Israeli and Palestinian patients. Arch Ophthalmol 127, 297-302, doi:10.1001/archophthalmol.2008.615 (2009).
- Gao, F. J. et al. Genetic and Clinical Findings in a Large Cohort of Chinese Patients with Suspected Retinitis Pigmentosa. Ophthalmology 126, 1549-1556, doi:10.1016/j.ophtha.2019.04.038 (2019).
- Alsalamah, A. K., Khan, A. O., Bakar, A. A., Schatz, P. & Nowilaty, S. R. Recognizable Patterns of Submacular Fibrosis in Enhanced S-Cone Syndrome. Ophthalmol Retina 5, 918-927, doi:10.1016/j.oret.2021.03.014 (2021).
- Habibi, I. et al. Different Phenotypes in Pseudodominant Inherited Retinal Dystrophies. Front Cell Dev Biol 9, 625560, doi:10.3389/fcell.2021.625560 (2021).
- Al-Khuzaei, S. et al. Novel Pathogenic Sequence Variants in NR2E3 and Clinical Findings in Three Patients. Genes (Basel) 11, doi:10.3390/genes11111288 (2020).
- de Carvalho, E. R. et al. Enhanced S-Cone Syndrome: Spectrum of Clinical, Imaging, Electrophysiologic, and Genetic Findings in a Retrospective Case Series of 56 Patients. Ophthalmol Retina 5, 195-214, doi:10.1016/j.oret.2020.07.008 (2021).
- Hebbar, P. et al. Genetic risk variants for metabolic traits in Arab populations. Sci Rep 7, 40988, doi:10.1038/srep40988 (2017).
- Van Cauwenbergh, C. et al. Mutations in Splicing Factor Genes Are a Major Cause of Autosomal Dominant Retinitis Pigmentosa in Belgian Families. PLoS One 12, e0170038, doi:10.1371/journal.pone.0170038 (2017).
- Kuniyoshi, K. et al. New truncation mutation of the NR2E3 gene in a Japanese patient with enhanced S-cone syndrome. Jpn J Ophthalmol 60, 476-485, doi:10.1007/s10384-016-0470-0 (2016).
- Kannabiran, C., Singh, H., Sahini, N., Jalali, S. & Mohan, G. Mutations in TULP1, NR2E3, and MFRP genes in Indian families with autosomal recessive retinitis pigmentosa. Mol Vis 18, 1165-1174 (2012).
- Udar, N., Small, K., Chalukya, M., Silva-Garcia, R. & Marmor, M. Developmental or degenerative–NR2E3 gene mutations in two patients with enhanced S cone syndrome. Mol Vis 17, 519-525 (2011).
- Naik, A. et al. Enhanced S-cone syndrome: Clinical spectrum in Indian population. Indian J Ophthalmol 67, 523-529, doi:10.4103/ijo.IJO_1480_18 (2019).
- Haider, N. B. et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet 24, 127-131, doi:10.1038/72777 (2000).
- Schorderet, D. F. & Escher, P. NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP). Hum Mutat 30, 1475-1485, doi:10.1002/humu.21096 (2009).
- Escher, P. et al. Mutations in NR2E3 can cause dominant or recessive retinal degenerations in the same family. Hum Mutat 30, 342-351, doi:10.1002/humu.20858 (2009).
- Stone, E. M. et al. Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology 124, 1314-1331, doi:10.1016/j.ophtha.2017.04.008 (2017).
- Iarossi, G. et al. A Novel Autosomal Recessive Variant of the NRL Gene Causing Enhanced S-Cone Syndrome: A Morpho-Functional Analysis of Two Unrelated Pediatric Patients. Diagnostics (Basel) 12, doi:10.3390/diagnostics12092183 (2022).
- Newman, H. et al. Homozygosity for a Recessive Loss-of-Function Mutation of the NRL Gene Is Associated With a Variant of Enhanced S-Cone Syndrome. Invest Ophthalmol Vis Sci 57, 5361-5371, doi:10.1167/iovs.16-19505 (2016).
- Nishiguchi, K. M. et al. Recessive NRL mutations in patients with clumped pigmentary retinal degeneration and relative preservation of blue cone function. Proc Natl Acad Sci U S A 101, 17819-17824, doi:10.1073/pnas.0408183101 (2004).
- Littink, K. W. et al. Autosomal Recessive NRL Mutations in Patients with Enhanced S-Cone Syndrome. Genes (Basel) 9, doi:10.3390/genes9020068 (2018).
- Haider, N. B., Naggert, J. K. & Nishina, P. M. Excess cone cell proliferation due to lack of a functional NR2E3 causes retinal dysplasia and degeneration in rd7/rd7 mice. Hum Mol Genet 10, 1619-1626, doi:10.1093/hmg/10.16.1619 (2001).
- Haider, N. B. et al. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp Eye Res 89, 365-372, doi:10.1016/j.exer.2009.04.006 (2009).
- Haider, N. B. et al. The transcription factor Nr2e3 functions in retinal progenitors to suppress cone cell generation. Vis Neurosci 23, 917-929, doi:10.1017/S095252380623027X (2006).
- Oh, E. C. et al. Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors. Brain Res 1236, 16-29, doi:10.1016/j.brainres.2008.01.028 (2008).
- Mears, A. J. et al. Nrl is required for rod photoreceptor development. Nat Genet 29, 447-452, doi:10.1038/ng774 (2001).
- Khan, A. O., Aldahmesh, M. & Meyer, B. The enhanced S-cone syndrome in children. BMJ Case Rep 2009, doi:10.1136/bcr.10.2008.1163 (2009).
- Fishman, G. A., Jampol, L. M. & Goldberg, M. F. Diagnostic features of the Favre-Goldmann syndrome. Br J Ophthalmol 60, 345-353, doi:10.1136/bjo.60.5.345 (1976).
- Garafalo, A. V. et al. Cone Vision Changes in the Enhanced S-Cone Syndrome Caused by NR2E3 Gene Mutations. Invest Ophthalmol Vis Sci 59, 3209-3219, doi:10.1167/iovs.18-24518 (2018).
- Yzer, S. et al. Expanded clinical spectrum of enhanced S-cone syndrome. JAMA Ophthalmol 131, 1324-1330, doi:10.1001/jamaophthalmol.2013.4349 (2013).
- Tsang, S. H. & Sharma, T. Enhanced S-Cone Syndrome (Goldmann-Favre Syndrome). Adv Exp Med Biol 1085, 153-156, doi:10.1007/978-3-319-95046-4_28 (2018).
- Vincent, A., Robson, A. G. & Holder, G. E. Pathognomonic (diagnostic) ERGs. A review and update. Retina 33, 5-12, doi:10.1097/IAE.0b013e31827e2306 (2013).
- Hood, D. C., Cideciyan, A. V., Roman, A. J. & Jacobson, S. G. Enhanced S cone syndrome: evidence for an abnormally large number of S cones. Vision Res 35, 1473-1481, doi:10.1016/0042-6989(95)98727-q (1995).
- Jacobson, S. G., Marmor, M. F., Kemp, C. M. & Knighton, R. W. SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci 31, 827-838 (1990).
- Marmor, M. F., Jacobson, S. G., Foerster, M. H., Kellner, U. & Weleber, R. G. Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am J Ophthalmol 110, 124-134, doi:10.1016/s0002-9394(14)76980-6 (1990).
- Audo, I. et al. Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci 49, 2082-2093, doi:10.1167/iovs.05-1629 (2008).
- Nakamura, M. et al. Enhanced S-cone syndrome with subfoveal neovascularization. Am J Ophthalmol 133, 575-577, doi:10.1016/s0002-9394(01)01428-3 (2002).
- Zerbib, J. et al. Retinochoroidal Anastomosis Associated with Enhanced S-Cone Syndrome. Retin Cases Brief Rep 13, 295-299, doi:10.1097/ICB.0000000000000594 (2019).
- Bazvand, F., Khojasteh, H. & Zarei, M. Novel findings in enhanced S-cone syndrome: a case with macular retinal neovascularization and severe retinal vasculitis. Doc Ophthalmol 139, 221-226, doi:10.1007/s10633-019-09706-6 (2019).
- Sharon, D., Sandberg, M. A., Caruso, R. C., Berson, E. L. & Dryja, T. P. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol 121, 1316-1323, doi:10.1001/archopht.121.9.1316 (2003).
- Chavala, S. H. et al. An Arg311Gln NR2E3 mutation in a family with classic Goldmann-Favre syndrome. Br J Ophthalmol 89, 1065-1066, doi:10.1136/bjo.2005.068130 (2005).
- Gire, A. I. et al. The Gly56Arg mutation in NR2E3 accounts for 1-2% of autosomal dominant retinitis pigmentosa. Mol Vis 13, 1970-1975 (2007).
- Bohrer, L. R. et al. Correction of NR2E3 Associated Enhanced S-cone Syndrome Patient-specific iPSCs using CRISPR-Cas9. Genes (Basel) 10, doi:10.3390/genes10040278 (2019).
- Iannaccone, A., Fung, K. H., Eyestone, M. E. & Stone, E. M. Treatment of adult-onset acute macular retinoschisis in enhanced s-cone syndrome with oral acetazolamide. Am J Ophthalmol 147, 307-312 e302, doi:10.1016/j.ajo.2008.08.003 (2009).
- Chatzistergiou, V. et al. Optical Coherence Tomography Analysis of Cystoid Macular Edema in Retinal Dystrophy Treated with Oral Acetazolamide: Two Cases. Klin Monbl Augenheilkd 237, 484-486, doi:10.1055/a-1068-2762 (2020).
- Genead, M. A., Fishman, G. A. & McAnany, J. J. Efficacy of topical dorzolamide for treatment of cystic macular lesions in a patient with enhanced S-cone syndrome. Doc Ophthalmol 121, 231-240, doi:10.1007/s10633-010-9247-9 (2010).
- Hajali, M. & Fishman, G. A. Dorzolamide use in the management of macular cysts in a patient with enhanced s-cone syndrome. Retin Cases Brief Rep 3, 121-124, doi:10.1097/ICB.0b013e31818faa21 (2009).
- Bechet, L. et al. Management of a case of Enhanced S-cone syndrome with massive foveoschisis treated with pars plana vitrectomy with silicone oil tamponade. Ophthalmic Genet 42, 615-618, doi:10.1080/13816810.2021.1925927 (2021).
- Broadhead, G. K., Grigg, J. R., McCluskey, P., Korsakova, M. & Chang, A. A. Bevacizumab for choroidal neovascularisation in enhanced S-cone syndrome. Doc Ophthalmol 133, 139-143, doi:10.1007/s10633-016-9555-9 (2016).
- Bertoli, F., Pignatto, S., Rizzetto, F. & Lanzetta, P. A 5-Year-Old Case of Choroidal Neovascularization in Enhanced S-Cone Syndrome Treated with Ranibizumab. Case Rep Ophthalmol 9, 510-515, doi:10.1159/000495743 (2018).
Faculty Approval by: Paul Bernstein, MD, PhD
Copyright: Copyright Ramshekar and Ronquillo, ©2023. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
Identifier: Moran_CORE_126913
Lyme Disease-Associated Uveitis: A Case Report and Review Emphasizing the Importance of Travel History and Geographic Considerations
Home / Intraocular Inflammation and Uveitis / Infectious Uveitis
Title: Lyme Disease-Associated Uveitis: A Case Report and Review Emphasizing the Importance of Travel History and Geographic Considerations
Authors: Andrew DesLauriers, BA; Marissa Larochelle, MD
Photographer: Rich Ordonez
Date: 08/09/2023
Keywords/Main Subjects: lyme disease, intermediate uveitis, anterior uveitis, travel history
Diagnosis: Lyme Disease-Associated Intermediate and Anterior Uveitis
Description of Case:
Introduction:
Lyme disease, caused by the Borrelia genus of bacteria transmitted through Ixodes ticks, is primarily reported in the Northeast and Mid-Atlantic regions of the United States, as well as in certain parts of Europe and Japan.1,2 Ocular involvement can occur at any stage of the disease, presenting with diverse manifestations, including uveitis. Early identification of Lyme-associated uveitis is crucial to initiate appropriate treatment and prevent long-term complications. Lyme-associated uveitis should be considered in the differential diagnosis of patients with new-onset uveitis and a history of travel to endemic areas.
Case Presentation:
In early August, a 10-year-old male presented to the Moran Eye Center in Utah with sharp, bilateral eye pain, blurred vision, and increased redness for three days. He reported a history of mild, intermittent eye redness without pain for three months prior to presentation. Additionally, one month before the increase in ocular symptoms, the patient experienced a brief generalized illness with fevers, headache, and cervical/post-cervical and submandibular lymphadenopathy, initially attributed to a self-limited viral illness. The patient, who had recently been diagnosed with autoimmune thyroiditis and a strong family history of autoimmune diseases, reported no previous ocular history. The patient’s mother reported that he regularly interacts with several stray cats near their home, but there were no instances of reported bites or scratches. Additionally, the patient and his family had vacationed in Vermont, a Lyme disease endemic area, three months prior to his presentation at the Moran Eye Center, though no tick bites were reported during their trip.
Examination and Diagnosis:
On examination, the patient had 1-2+ mixed cells in the anterior chamber and 1+ cell and 2+ vitreous haze bilaterally with notable vitreous debris. Fluorescein angiography revealed mild diffuse vascular leakage in both eyes. Based on the clinical picture, the patient was diagnosed with bilateral, simultaneous anterior and intermediate uveitis. Initial laboratory workup was significant for positive Bartonella IgM (1:256) but negative Bartonella IgG. Consequently, the patient was treated for presumed Bartonella infection with doxycycline and rifampin, along with topical prednisolone, cyclopentolate, and an oral prednisone taper to manage inflammation and prevent complications. The patient was referred to Infectious Disease clinic and further history taking revealed hiking in Lyme-endemic Vermont, so Lyme serology was added to the lab work up.
Treatment and Follow-Up:
Two weeks later, additional lab results indicated a positive Lyme ELISA and Immunoblot assay. While the initial Bartonella IgM had been elevated, a repeat Bartonella IgM test was negative (1:64), and two Bartonella IgG assays were also both negative, indicating that the initial positive IgM was likely a false-positive. Fortunately, doxycycline is effective in the treatment of both Bartonella and Lyme Borreliosis, so the patient had already been started on an effective regimen. Rifampin was discontinued, and the patient continued doxycycline for an additional 6 weeks. Following a two-month course of doxycycline with an oral prednisone taper, the patient’s symptoms had completely resolved, and a clinical examination showed no remaining signs of intraocular inflammation. The patient remained symptom-free, with no intraocular inflammation or recurrences at 11 months after the initial presentation.
Lyme Disease Overview:
The Borrelia genus of bacteria, transmitted through a bite from an Ixodes tick, cause Lyme disease, which progresses through three clinical stages. Stage 1 is characterized by a bulls-eye rash (Erythema Migrans) at the infection site in around 80% of patients, accompanied by constitutional flu-like symptoms in some cases. Stage 2 involves hematogenous spread to organs, leading to a variety of sequalae, including meningitis, neuropathies, and acute carditis. Stage 3 presents months to years post-inoculation with chronic manifestations, most commonly oligoarticular arthritis. Notably, 2-3% of Lyme disease patients present with Stage 2 or Stage 3 sequelae within days of exposure.3 Infection with Borrelia tends to peak during the late spring or early summer in endemic areas.3
Ocular Involvement in Lyme Disease:
Ocular involvement, typically bilateral, can occur at any disease stage.4 The most common ocular symptom associated with Lyme disease is a follicular conjunctivitis, occurring in approximately 11% of patients most commonly during Stage 1 of disease.5 Stages 2 and 3 of Lyme disease are associated with a variety of symptoms including all types of uveitis, retinal vasculitis, neuroretinis, episcleritis, keratitis, papillitis, optic neuritis, and cranial nerve palsies of nerves III, V, VI, and VII.4,6
Lyme-Associated Uveitis:
Lyme uveitis, an uncommon manifestation of Stage 2 and Stage 3 Lyme disease, comprises up to 4.3% of all uveitis cases at referral centers in endemic areas; however, the reported prevalence varies widely and is much lower in non-endemic areas.4,7 Patients typically present with symptoms such as eye pain, redness, blurred vision, new floaters, and photophobia. The most common presentation is an intermediate uveitis; however, cases of anterior, posterior, and panuveitis have also been reported.4 Uveitis is commonly accompanied by retinal vasculitis. The uveitis can be granulomatous or non-granulomatous in nature.8 Published reports have described varied clinical findings of Lyme uveitis in different patients, with some demonstrating choroidal neovascularization and others showing multifocal white dots in the posterior pole, resembling white dot syndromes such as acute posterior multifocal placoid pigment epitheliopathy (APMPPE).9,10,11
Diagnosis and Management:
Lyme-associated uveitis should be considered in any patient presenting with new-onset uveitis after travel to a Lyme-endemic area. A detailed history should focus on possible exposure and any symptoms outside of eye-related issues, though ocular involvement can be the sole manifestation of Lyme infection, meaning patients may not have other systemic symptoms.4,6 Particular attention should be paid to patients who report spending any amount of time outdoors in an endemic region, engaging in activities such as hiking, gardening, hunting, or forestry work.3
Tissue biopsies are seldom useful in the detection of ocular Borrelia infection.4 Routine screening for Lyme disease in all uveitis patients isn’t recommended due to the low incidence of Lyme-associated uveitis. However, in the right clinical context and with a history of travel to Lyme-endemic areas, serum antibody testing can be used to confirm the diagnosis. Lyme-associated uveitis management should include regular surveillance, and multi-modal imaging is useful for monitoring progression and treatment response.11
Treatment:
Borrelis burgdorferi, the species of bacteria causing Lyme disease in the United States, is typically sensitive to tetracyclines and many B-lactam antibiotics. Doxycycline, ceftriaxone, and amoxicillin can treat any stage of disease, with ceftriaxone preferred for CNS involvement.3,4 These medications are also effective in treating ocular disease, with some studies suggesting use of a cephalosporin for better ocular penetration.12 Steroid treatment alone does not worsen disease but is insufficient in resolving ocular complications.4 Lyme-associated uveitis typically requires a combined approach of both antibiotic treatment and management of inflammation.
Discussion:
This case illustrates the importance of maintaining a high suspicion for Lyme disease in patients with uveitis if there is a report of recent travel to Lyme-endemic areas. Conversely, it is important to note that Lyme disease is a less likely cause of uveitis in patients without exposure to an endemic region, for example, residents of Utah who have not travelled out of the state. While there are small populations of ticks in Utah that could potentially carry Lyme disease, tests conducted on samples of these ticks have not detected the presence of Borrelia bacteria.13 Even in endemic areas, Lyme disease is an uncommon cause of uveitis, however it should be considered in patients with the appropriate risk factors such as a history of time spent outdoors and additional clinical symptoms. Please see the geographic distributions of reported Lyme disease included in the resources section below to help guide risk stratification based on travel history.
Lyme-associated uveitis can present in various forms, making it crucial to include this infectious etiology in the differential diagnosis when risk factors are present and perform appropriate antibody testing for confirmation. In this case, the patient’s travel to an endemic area in late spring placed him at risk of Lyme exposure. His intermittent eye redness preceding the acute uveitis episode was likely attributable to the follicular conjunctivitis often reported in Lyme disease’s Stage 1. Furthermore, the febrile illness he experienced one month prior to presenting at the Moran Eye Center was likely a systemic manifestation of Lyme disease. Early identification allows for timely treatment and prevents vision-threatening complications.14 Untreated Lyme uveitis, although rare, can result in complete loss of vision and phthisis bulbi.15
Conclusion:
Lyme-associated uveitis, though uncommon, can lead to serious complications. This case report emphasizes the significance of clinical acumen in diagnosing and treating vision-threatening diseases related to Lyme borreliosis. Awareness of Lyme disease’s geographic distribution and risk factors can guide risk stratification based on travel history, helping to identify cases promptly and initiate appropriate management. Clinicians in non-endemic areas should maintain a high index of suspicion for Lyme uveitis in patients with relevant travel history, particularly in the setting of characteristic systemic symptoms.
Images or video:

Figure 1: Lyme Disease Map. Distribution of reported lyme disease cases in the United States in 2021. Each green dot represents a case of Lyme Disease reported to the CDC in 2021. States shaded with light blue are regarded as “high incidence states” by the CDC. States shaded in grey are considered low incidence. Lyme Disease Map acquired from the CDC website: https://www.cdc.gov/lyme/datasurveillance/lyme-disease-maps.html

Figure 2. Fluorescein angiography images of the patient’s right eye that were taken upon presentation. The images captured at 0:35 (top) and 5:24 (bottom) after infusion reveal mild diffuse vascular leakage.
Summary of the Case:
Lyme disease, caused by the Borrelia genus of bacteria and transmitted by Ixodes ticks, can lead to a variety of ocular complications including uveitis. Transmission typically occurs in endemic regions with the appropriate ecological factors to allow for bacterial reproduction and transmission. A 10-year-old male presented with eye pain, redness, and blurred vision after vacationing in a Lyme-endemic region, although he had no reported tick bites. His examination and subsequent tests revealed Lyme-associated uveitis. The importance of early identification and treatment is underscored, as untreated cases can result in severe vision complications. Clinicians in non-endemic regions should maintain vigilance for Lyme uveitis in patients with relevant travel history in order to ensure prompt and effective management.
Format: Case Report, Literature Review
References
- Lyme Disease Map | Lyme Disease | CDC. Accessed August 1, 2023. https://www.cdc.gov/lyme/datasurveillance/lyme-disease-maps.html
- Overview | Johns Hopkins Lyme and Tickborne Diseases Dashboard. Accessed August 1, 2023. https://www.hopkinslymetracker.org/overview/
- Steere AC, Strle F, Wormser GP, et al. Lyme borreliosis. Nature Reviews Disease Primers. 2016;2(1):16090. doi:10.1038/nrdp.2016.90
- Bernard A, Seve P, Abukhashabh A, et al. Lyme-associated uveitis: Clinical spectrum and review of literature. Eur J Ophthalmol. 2020;30(5):874-885. doi:10.1177/1120672119856943
- Steere AC, Bartenhagen NH, Craft JE, et al. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983;99(1):76-82. doi:10.7326/0003-4819-99-1-76
- Lesser RL. Ocular manifestations of Lyme disease. The American Journal of Medicine. 1995;98(4, Supplement 1):60S-62S. doi:10.1016/S0002-9343(99)80045-X
- Mikkilä H, Seppälä I, Leirisalo-Repo M, Immonen I, Karma A. The etiology of uveitis: The role of infections with special reference to Lyme borreliosis. Acta Ophthalmologica Scandinavica. 1997;75(6):716-719. doi:10.1111/j.1600-0420.1997.tb00637.x
- Mikkilä HO, Seppälä IJT, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-587. doi:10.1016/S0161-6420(99)00128-1
- Kılıç Müftüoğlu İ, Aydın Akova Y, Gür Güngör S. A Case of Lyme Disease Accompanied by Uveitis and White Dot Syndrome. Turk J Ophthalmol. 2016;46(5):241-243. doi:10.4274/tjo.25991
- Amer R, Brannan S, Forrester JV. Inflammatory choroidal neovascular membrane in presumed ocular Lyme borreliosis. Acta Ophthalmol. 2009;87(3):346-348. doi:10.1111/j.1755-3768.2007.01160.x
- Ferro Desideri L, Rosa R, Forte P, et al. Multimodal imaging for the management of Lyme-associated uveitis: A case report from an Italian tertiary center. European Journal of Ophthalmology. Published online January 29, 2023:11206721231154172. doi:10.1177/11206721231154172
- Lindström BE, Skogman BH, Lindström AK, Tallstedt L, Nilsson K. Borrelia Ocular Infection: A Case Report and a Systematic Review of Published Cases. Ophthalmic Res. 2022;65(2):121-130. doi:10.1159/000521307
- Richardson K, Davis R, Ramirez R. Ticks and Tick-Borne Diseases of Utah. Utah State University Extension and Utah Plant Pest Diagnostic Laboratory; 2023.
- Copeland RAJ. Lyme uveitis. Int Ophthalmol Clin. 1990;30(4):291-293. doi:10.1097/00004397-199030040-00019
- Kauffmann DJ, Wormser GP. Ocular Lyme disease: case report and review of the literature. British Journal of Ophthalmology. 1990;74(6):325-327. doi:10.1136/bjo.74.6.325
Faculty Approval by: Marissa Larochelle, MD
Copyright: Andrew DesLauriers, ©2023. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
Identifier: Moran_CORE_126899
Iridocorneal Endothelial (ICE) Syndrome
Title: Iridocorneal Endothelial (ICE) Syndrome
Author: Krishna Mallem, Drexel University College of Medicine, MD Class of 2024; . Austin Nakatsuka, MD
Date: July 2023
Keywords/Main Subjects: Iridocorneal endothelial syndrome; ICE; Gonioscopy assisted transluminal trab
- Electron microscopy of corneal endothelium in ICE syndrome.
- Polycoria, corectopia and atrophic iris changes in a patient.
eculotomy; GATT
Introduction:
Iridocorneal endothelial (ICE) syndrome is an unusual disorder of the eye that involves an abnormality of the corneal endothelium. ICE syndrome typically affects middle aged women, and occurs sporadically and unilaterally 1,2. It consists of three clinical variants, which are Chandler syndrome, essential iris atrophy, and iris nevus or Cogan-Reese Syndrome. The distinction between these variants is based on clinical exam findings. Abnormalities of the corneal endothelium in ICE syndrome can lead to varying degrees of corneal edema, iris atrophy, and secondary angle closure glaucoma. This condition can be potentially blinding due to glaucoma recalcitrant to conventional therapies and recurrent corneal edema1-4. We present a case of a patient with ICE syndrome who undergoes gonioscopy assisted transluminal trabeculotomy (GATT) for uncontrolled IOP on medical therapy.
Photos taken from Moran Eye Center archives.
Pathophysiology:
Similar to corneal guttata found in Fuchs endothelial dystrophy, the corneal endothelium in patients with ICE syndrome is described to have a “beaten bronze” appearance. Scanning and tunneling electron microscopy of endothelial cells in patients with ICE syndrome reveal a population of well differentiated epithelial-like cells in place of normal endothelium. This cell population displays migratory characteristics, and is believed to migrate posteriorly onto the trabecular meshwork and the iris. As this tissue contracts within the angle and the iris, peripheral anterior synechiae (PAS) may form, and the iris may undergo morphological changes. Contraction of tissue can also predispose patients to secondary angle closure glaucoma. Corneal edema can develop as a consequence of elevated intraocular pressure as well as due to failure of the normal pump function of the dysfunctional endothelium3-8.
Case presentation:
Our patient is a 51 year old male who presented in February 2017 for glaucoma evaluation in the left eye (OS). IOP on examination was 34. Corneal pachymetry showed a corneal thickness of 523 microns OS. Gonioscopy revealed a broad area of peripheral anterior synechiae (PAS) temporally, with smaller areas of PAS inferiorly. Slit lamp exam was significant for diffuse endothelial guttae-like changes in the cornea with an oval shaped pupil. IOP and slit lamp exam of the right eye were unremarkable. Baseline visual fields were obtained and showed scattered defects in both eyes. Baseline optical coherence tomography (OCT) of the retinal nerve fiber layer (RNFL) revealed normal thickness in both eyes. Given examination findings at the baseline visit, the patient was diagnosed with ICE syndrome and was started on latanoprost once daily OS for intraocular pressure control.
The patient was monitored regularly and over the course of several years his medical therapy was escalated due to persistently high IOPs as well as changes on field and OCT testing. In March 2022, the patient was on maximal medical therapy with netarsudil-latanoprost, dorzolamide-timolol, and brimonidine in the left eye. Despite this, his IOP was 37 on the left. Slit lamp examination at this time showed a distorted pupil with high PAS temporally. Visual field testing showed a subtle superior arcuate scotoma OS. OCT RNFL showed significant thinning inferiorly and superiorly OS. The decision to proceed with surgery was made given the patient’s young age and long term prognosis due to medically uncontrolled IOP.
Patient underwent gonioscopy assisted transluminal trabeculotomy (GATT) with anterior goniosynechiolysis without cataract extraction in May of 2022. After successful surgery without complications, the patient’s iop quickly normalized and was maintained over a year later on two drops. OCT findings 6 months after surgery revealed stability, however, there were signs of progression 1 year following the procedure, despite well controlled IOPs. Additionally, the patient has recently developed corneal guttae and edema which is currently being treated medically with hypertonic . Although the patient’s IOP continues to be well controlled, the current focus is now on controlling corneal edema, with consideration for corneal transplantation in the future if the cornea does not recover with medical therapy alone.
Discussion:
This is a unique case of a patient with ICE syndrome who was managed with GATT and goniosynechiolysis in the left eye for uncontrolled IOP and progressive glaucomatous changes. To our knowledge, there is not another case of GATT or other microinvasive glaucoma surgery (MIGS) being performed in a patient with ICE syndrome in the literature. It is well documented that filtering procedures in ICE syndrome are only modestly successful at 5 years (53% with drainage device and 29% with trabeculectomy)⁸ and often need to be repeated to obtain IOP control. Although GATT has successfully controlled IOP one year out from surgery, there is still evidence of OCT progression and further surgical treatment may be necessary. Furthermore, the patient has developed more clinically significant corneal edema and guttae, suggesting corneal decompensation, which may require surgical intervention with a corneal transplant. This case highlights the difficulty in managing patients with ICE syndrome and documents the novel use of GATT for IOP control in ICE syndrome.
References:
- Laganowski, H. C., Muir, M. G. K., & Hitchings, R. A. (1992). Glaucoma and the iridocorneal endothelial syndrome. Archives of Ophthalmology, 110(3), 346-350.
- Dubey, S., Jain, K., Singh, S., & Mukhejee, S. (2021). Iridocorneal Endothelial Syndrome with Coexisting Macular Edema and Neurosensory Detachment: An Unusual Case Report. Journal of Current Glaucoma Practice, 15(3), 149.
- Campbell, D. G., Shields, M. B., & Smith, T. R. (1978). The corneal endothelium and the spectrum of essential iris atrophy. American journal of ophthalmology, 86(3), 317-324.
- Basic and Clinical Science Course (BCSC). American Academy of Ophthalmology, (2010-2011), 142-144.
- Eagle, R. C., Font, R. L., Yanoff, M., & Fine, B. S. (1979). Proliferative endotheliopathy with iris abnormalities: the iridocorneal endothelial syndrome. Archives of Ophthalmology, 97(11), 2104-2111.
- Doan A, Alward W: Iridocorneal Endothelial Syndrome (ICE) – essential iris atrophy : 63-year-old female with PAS, “iris mass”, corectopia, and increased IOP OS. February 21, 2005; Available from: http://www.EyeRounds.org/cases/case14.htm.
- Levy, S. G., Kirkness, C. M., Moss, J., Ficker, L., & McCartney, A. C. (1996). The histopathology of the iridocorneal-endothelial syndrome. Cornea, 15(1), 46-54.
- Doe, E. A., Budenz, D. L., Gedde, S. J., & Imami, N. R. (2001). Long-term surgical outcomes of patients with glaucoma secondary to the iridocorneal endothelial syndrome. Ophthalmology, 108(10), 1789-1795.