Ocular cicatricial pemphigoid: summary of pathogenesis, diagnosis & treatment
Home / External Disease and Cornea / Diagnosis and Management of Immune-Related Disorders of the External Eye
Title: Ocular Cicatricial Pemphigoid: summary of pathogenesis, diagnosis & treatment
Authors: Nicholas J. Auteri, Colin Ip, Brian E. Zaugg, Mark D. Mifflin
Date: 09/25/2021
Keywords/Main Subjects: Ocular Cicatricial Pemphigoid (OCP), Mucus Membrane Pemphigoid (MMP)
Diagnosis: Ocular Cicatricial Pemphigoid
Description of Case: Ocular cicatricial pemphigoid (OCP) is the ocular manifestation of mucus membrane pemphigoid (MMP), characterized by chronic conjunctivitis with scarring.
Images:
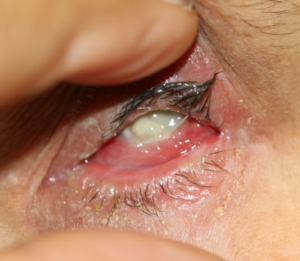
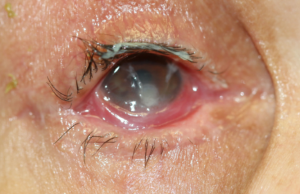
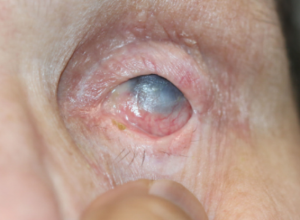
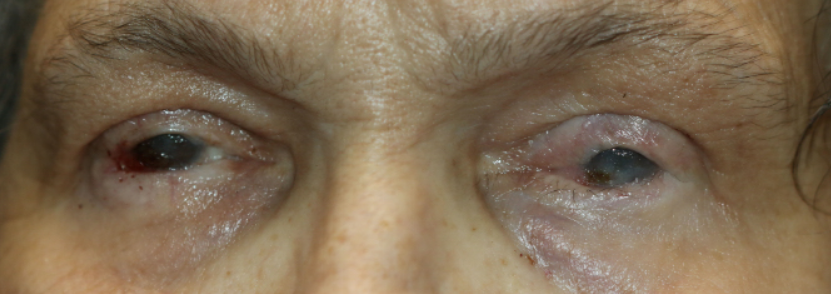
All images are from the same patient with OCP. She progressed through all four stages of OCP due to poor treatment compliance and follow-up.
Figure 1: Initial presentation of the patient with Stage I OCP, characterized by chronic conjunctivitis.
Figure 2: Follow-up photographs of the patient with an acute exacerbation of OCP.
Figure 3: One-week follow-up photographs monitoring treatment of acute OCP exacerbation.
Figure 4: Uncontrolled, recurrent OCP at the nine-month follow-up visit.
Figure 5 & 6: Stage II OCP, characterized by shortening of the inferior fornix.
Figure 7: Another photograph of uncontrolled, recurrent OCP demonstrating corneal keratinization.
Figure 8: Additional photographs of stage III OCP.
Figure 9: Stage IV OCP, characterized by ankyloblepharon.
Summary of the Case:
Introduction:
Mucus membrane pemphigoid (MMP) is a chronic, blistering autoinflammatory disease of the mucosal surfaces of the body due to linear deposition of IgG, IgA, IgM and/or C3 in the basement membrane of mucosal epithelia. The condition may affect oral, ocular, pharyngeal, laryngeal, genital and/or anal mucosa. Ocular cicatricial pemphigoid (OCP) is the name given to MMP that manifests in the eye with cicatrizing conjunctivitis.
OCP leads to vision loss secondary to keratopathy with scarring and neovascularization. The natural history of untreated OCP is progression to end-stage, bilateral blindness over 10-30 years, however some cases progress more rapidly.1 Approximately one-third of patients will experience treatment-free remission at some point in the natural history of the disease.2,3 However, treatment generally requires systemic immunosuppression with the help of a rheumatologist to prevent progression of ocular disease and subsequent vision loss.
Epidemiology:
The estimated incidence of OCP is between 1/8,000 and 1/46,000.4-7 Most patients are between 60 and 70 years-old at the time of diagnosis.1,8,9 OCP has a female predominance with an estimated F:M ratio of 1.5:1 to 3:1.1
Pathophysiology:
The pathogenesis of OCP is due to a systemic autoimmune disorder of autoantibodies directed against mucosal epithelial basement membrane antigens. The literature has identified five potential antigenic targets in OCP: alpha-6 beta-4 integrin (hemidesmosome component)10-12, laminin 513,14, bullous pemphigoid antigen 2 (BP180)15,16, unspecified 168-kDa and 45-kDa antigens17,18.
Regardless of the true antigenic target, OCP causes a scarring conjunctivitis through a three-step process. First, autoantibodies bind to the antigenic target in the mucosal basement membrane, which leads to an inflammatory cascade characterized by cytokine secretion and white blood cell recruitment. Second, these newly recruited white blood cells secrete more proinflammatory cytokines (including TGF-beta and IFN-gamma) to maintain the inflammatory response to autoantibody binding. Third, TGF-beta and IFN-gamma cause conjunctival scarring and the clinical manifestations of OCP.19-21
A two-hit hypothesis leading to the production of autoantibodies has been proposed for the pathogenesis of OCP.1 First, an underlying genetic susceptibility increases the risk of developing OCP. Second, some environmental insult triggers an inflammatory cascade that unmasks the underlying genetic susceptibility to OCP, and clinical signs and symptoms become apparent. The most likely genetic predisposition to OCP is the allele HLA DQw7 (HLA DQB1*0301).22,23 A wide variety of medications and microbes have been proposed as the environmental insult that unmasks OCP, but none have provided sufficiently convincing evidence.24-31 An epitope spreading theory has also been put forward, which states that conjunctival inflammation leads to unmasking and immune system detection of basement membrane antigens and a subsequent autoinflammatory response.32 This theory has been used to support the finding that OCP may occur as a sequela of Stevens-Johnson syndrome (SJS).33
Clinical findings/Diagnosis:
The clinical findings of OCP are variable. Often, patients present with monocular, relapsing-remitting conjunctivitis. Typically, these patients progress to bilateral disease and symblepharon formation in the inferior fornix with 5 years of symptom-onset.1,34,35 Trichiasis is also common secondary to shortening of the posterior lamella. Severe OCP leads to ankyloblepharon (fusion of the upper and lower lids). Ultimately, vision is lost due to corneal keratinization and neovascularization secondary to the loss of lacrimal glands and conjunctival goblet cells.1 Extraocular manifestations of MMP are common in OCP patients. About 40% of OCP patients will have oral lesions, and 16% will have skin lesions.34
OCP is staged clinically using Foster’s classification system, and this staging system is used to determine treatment (discussed below)1,36:
- Stage I: chronic conjunctivitis with subepithelial fibrosis
- Stage II: shortening of the inferior fornix
- Stage IIa: 0-25% loss of inferior fornix depth
- Stage IIb: 25-50% loss of inferior fornix depth
- Stage IIc: 50-75% loss of inferior fornix depth
- Stage IId: 75-100% loss of inferior fornix depth
- Stage III: symblephara formation
- Stage IIIa: 0-25% horizontal involvement of sympblephara
- Stage IIIb: 25-50% horizontal involvement of sympblephara
- Stage IIIc: 50-75% horizontal involvement of sympblephara
- Stage IIId: 75-100% horizontal involvement of sympblephara
- Stage IV: ankyloblepharon and corneal keratinization
The diagnosis of OCP is usually established based on the clinical characteristics discussed above. The gold standard for OCP diagnosis, however, requires biopsy and immunohistochemistry (IHC). For best biopsy results, fresh frozen tissue should be sent for IHC. Fresh tissue is required for immunostaining, however, tissue fixed in formalin may also be sent if histology is desired. A positive IHC in OCP shows linear deposition of IgG, IgA, IgM and/or C3 at the basement membrane. Extraocular (i.e., skin) biopsy is preferred over conjunctival biopsy if there are active extraocular lesions, because conjunctival biopsy increases the risk of OCP exacerbation.32,34 Biopsy is often NOT required in cases with classic clinical features. Additionally, IHC is frequently falsely negative in OCP (sensitivity ~50%).37 Therefore, negative IHC cannot be used to rule-out OCP. If IHC is negative but there is a high clinical suspicion of OCP, an immunoperoxidase assay can be performed, which has a much higher sensitivity of 83% for OCP.37
The differential diagnosis for OCP is widely variable, including pseudopemphigoid, linear IgA bullous dermatitis, epidermolysis bullosa acquisita, Stevens-Johnson syndrome, toxic epidermal necrolysis, lupus, paraneoplastic disease, ocular surface squamous neoplasia, porphyria and scleroderma. A combination of the clinical history, clinical manifestations and patient risk factors should be used to narrow the differential. If the diagnosis remains unclear clinically, biopsy and IHC or immunoperoxidase assay should be undertaken. The differential is further complicated by the fact that a positive IHC cannot rule-out some of these alternate diagnoses, including linear IgA bullous dermatitis and epidermolysis bullosa acquisita.32
Treatment:
Once the diagnosis of OCP has been made, treatment should begin as soon as possible. The mainstay of OCP treatment is systemic immunosuppression. Topical immunosuppression and other topical treatments have little to no effect in OCP, but supportive topical therapy is often used, including preservative-free artificial tears for concurrent dry eye disease.1,38-40 Before delving into specific treatment options, it is important to note that many clinicians differ in their opinions on when OCP can be considered sufficiently treated and immunosuppression can be tapered. A good rule of thumb is to attempt tapering immunosuppression once ocular inflammation has been quiescent for 2 years. Disease progression can be insidious and therefore photograph documentation can help determine if the condition is responding to therapy. In addition to immunosuppression, OCP treatment also involves management of dry eye disease (DED) and trichiasis. DED should be managed with preservative-free artificial tears and/or punctal plugs/cautery.34 Trichiasis can be managed with manual eyelash removal at the slit-lamp or with more permanent solutions, such as cryoepilation or electrolysis.34,41 Keep in mind, however, that cryoepilation and electrolysis increase the risk of OCP progression and should only be performed if the disease is well controlled.
As mentioned above, specific treatments for OCP are selected based on disease severity. These treatment options are detailed below.
Mild to moderate OCP:
- Dapsone is the standard initial therapy for OCP. Its efficacy has been reported around 70%.1 Standard dosing is 50-200 mg/day. Alternate therapy should be considered if there is no response to dapsone after 12 weeks.32,42 Adverse events include hemolytic anemia in G6PD deficiency, leukopenia, aplastic anemia and methemoglobinemia.43,44 Patients should be screened for G6PD deficiency prior to beginning dapsone.
- Methotrexate is an alternative therapy if dapsone is ineffective or intolerable. Standard dosing is 5-25 mg once per week.45,46 Adverse events include GI distress, buccal ulcers, myelosuppression, pulmonary fibrosis and renal failure. Adverse events can be reduced by co-administration of folic acid.
- Mycophenolate mofetil (MMF) is another alternate therapy for mild to moderate OCP. Standard dosing is 500-1000 mg BID.47,48 Adverse events include GI distress and cytopenias.
- Azathioprine is another alternate therapy. Adverse events include bone marrow suppression. Prior to initiation of azathioprine, thiopurine methyltransferase (TPMT) levels should be checked to assess for risk of bone marrow suppression.8,47,49,50
Severe or refractory OCP:
- Cyclophosphamide with prednisone is the standard treatment of severe and refractory OCP.8,9,32,51,52 Cylophosphamide is dosed at 2 mg/kg/day and prednisone is dosed at 1 mg/kg/day for one week followed by a gradual taper to 0.25 mg/kg/day over 12 weeks and then discontinued.1 The reason that prednisone is used in addition to cyclophosphamide is because cyclophosphamide requires 6-8 weeks for a response to be seen in OCP.1 Adverse events include leukopenia (expected), GI distress, hemorrhagic cystitis, bladder cancer, infection, alopecia and infertility. Due to expected leukopenia, CBC should be monitored weekly for the first 3 months of treatment, and then monthly after that.53 Cyclophosphamide should be titrated to maintain WBC ≥3000/mm3, ANC >1000/mm3 and plt count >70k/uL. The adverse events and toxicity of cyclophosphamide require the treatment duration to be limited to less than one year.
- Intravenous immune globin (IVIG) is an alternative treatment option for severe and refractory OCP.54-57 The use of IVIG is limited due to its high cost. Adverse events include hypersensitivity reactions, vasculitis, increased risk of thrombosis, aseptic meningitis and renal failure.
- Rituximab is a monoclonal antibody to CD20 on B cells and can be used as an alternative therapy for severe or refractory OCP.58-62 Recommended dosing is 375 mg/m2 once per week for 4 weeks over 1-2 cycles.60 Rituximab carries a black box warning for multifocal leukoencephalopathy. Other adverse events include fatal infections, GI distress, serum sickness-like reaction and infusion reactions.
Finally, OCP patients may need ocular surgery to manage complications of the disease. These surgeries include, but are not limited to, amniotic membrane transplantation, symblepharon removal, entropion repair, penetrating keratoplasty, ocular surface stem cell transplantation or keratoprosthesis.34 As mentioned earlier, these surgical treatments should be deferred until OCP is well controlled on systemic immunosuppression due to the risk of OCP exacerbation after ocular surgery.
References:
- Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc 1986; 84:527.
- Foster CS, Neumann R, Tauber J. Long-term results of systemic chemotherapy for ocular cicatricial pemphigoid. Doc Ophthalmol 1992; 82:223.
- Neumann R, Tauber J, Foster CS. Remission and recurrence after withdrawal of therapy for ocular cicatricial pemphigoid. Ophthalmology 1991; 98:858.
- Bettelheim H, Kraft D, Zehetbauer G. [On the so-called ocular pemphigus (pemphigus ocularis, pemphigus conjunctivae)]. Klin Monbl Augenheilkd 1972; 160:65.
- Hardy WF, Lamb HD. Essential shrinkage of the conjunctiva with report of two cases. Am J Ophthalmol 1917; 34:289.
- Smith RC, Myers EA, Lamb HD. Ocular and oral pemphigus: Report of case with anatomic findings in eyeball. Arch Ophthalmol 1934; 11:635.
- BEDELL AJ. OCULAR PEMPHIGUS: A CLINICAL PRESENTATION OF KODACHROMES. Trans Am Ophthalmol Soc 1964; 62:109.
- Tauber J, Sainz de la Maza M, Foster CS. Systemic chemotherapy for ocular cicatricial pemphigoid. Cornea 1991; 10:185.
- Thorne JE, Woreta FA, Jabs DA, Anhalt GJ. Treatment of ocular mucous membrane pemphigoid with immunosuppressive drug therapy. Ophthalmology 2008; 115:2146.
- Chan RY, Bhol K, Tesavibul N, et al. The role of antibody to human beta4 integrin in conjunctival basement membrane separation: possible in vitro model for ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci 1999; 40:2283.
- Chan RY, Bhol K, Tesavibul N, et al. The role of antibody to human beta4 integrin in conjunctival basement membrane separation: possible in vitro model for ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci 1999; 40:2283.
- Kumari S, Bhol KC, Simmons RK, et al. Identification of ocular cicatricial pemphigoid antibody binding site(s) in human beta4 integrin. Invest Ophthalmol Vis Sci 2001; 42:379.
- Fujimoto W, Toi Y, Okazaki F, et al. Anti-epiligrin cicatricial pemphigoid with IgG autoantibodies to the beta and gamma subunits of laminin 5. J Am Acad Dermatol 1999; 40:637.
- Kirtschig G, Marinkovich MP, Burgeson RE, Yancey KB. Anti-basement membrane autoantibodies in patients with anti-epiligrin cicatricial pemphigoid bind the alpha subunit of laminin 5. J Invest Dermatol 1995; 105:543.
- Bédane C, McMillan JR, Balding SD, et al. Bullous pemphigoid and cicatricial pemphigoid autoantibodies react with ultrastructurally separable epitopes on the BP180 ectodomain: evidence that BP180 spans the lamina lucida. J Invest Dermatol 1997; 108:901.
- Balding SD, Prost C, Diaz LA, et al. Cicatricial pemphigoid autoantibodies react with multiple sites on the BP180 extracellular domain. J Invest Dermatol 1996; 106:141.
- Ghohestani RF, Nicolas JF, Rousselle P, Claudy AL. Identification of a 168-kDa mucosal antigen in a subset of patients with cicatricial pemphigoid. J Invest Dermatol 1996; 107:136.
- Smith EP, Taylor TB, Meyer LJ, Zone JJ. Identification of a basement membrane zone antigen reactive with circulating IgA antibody in ocular cicatricial pemphigoid. J Invest Dermatol 1993; 101:619.
- Razzaque MS, Foster CS, Ahmed AR. Role of connective tissue growth factor in the pathogenesis of conjunctival scarring in ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci 2003; 44:1998.
- Elder MJ, Dart JK, Lightman S. Conjunctival fibrosis in ocular cicatricial pemphigoid–the role of cytokines. Exp Eye Res 1997; 65:165.
- Lambiase A, Micera A, Mantelli F, et al. T-helper 17 lymphocytes in ocular cicatricial pemphigoid. Mol Vis 2009; 15:1449.
- Haider N, Neuman R, Foster CS, Ahmed AR. Report on the sequence of DQB1*0301 gene in ocular cicatricial pemphigoid patients. Curr Eye Res 1992; 11:1233.
- Ahmed AR, Foster S, Zaltas M, et al. Association of DQw7 (DQB1*0301) with ocular cicatricial pemphigoid. Proc Natl Acad Sci U S A 1991; 88:11579.
- Patten JT, Cavanagh HD, Allansmith MR. Induced ocular pseudopemphigoid. Am J Ophthalmol 1976; 82:272.
- Hirst LW, Werblin T, Novak M. Drug induced cicatrizing conjunctivitis simulating ocular pemphigoid. Cornea 1982; 1:121.
- Rahi AH, Chapman CM, Garner A, Wright P. Pathology of practolol-induced ocular toxicity. Br J Ophthalmol 1976; 60:312.
- Gibran SK. Unilateral drug-induced ocular pseudopemphigoid. Eye (Lond) 2004; 18:1270.
- Fiore PM, Jacobs IH, Goldberg DB. Drug-induced pemphigoid. A spectrum of diseases. Arch Ophthalmol 1987; 105:1660.
- Pouliquen Y, Patey A, Foster CS, et al. Drug-induced cicatricial pemphigoid affecting the conjunctiva. Light and electron microscopic features. Ophthalmology 1986; 93:775.
- Butt Z, Kaufman D, McNab A, McKelvie P. Drug-induced ocular cicatricial pemphigoid: a series of clinico-pathological reports. Eye (Lond) 1998; 12 ( Pt 2):285.
- Bhol K, Mohimen A, Neumann R, et al. Differences in the anti-basement membrane zone antibodies in ocular and pseudo-ocular cicatricial pemphigoid. Curr Eye Res 1996; 15:521.
- Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol 2002; 138:370.
- Chan LS, Soong HK, Foster CS, et al. Ocular cicatricial pemphigoid occurring as a sequela of Stevens-Johnson syndrome. JAMA 1991; 266:1543.
- Ahmed M, Zein G, Khawaja F, Foster CS. Ocular cicatricial pemphigoid: pathogenesis, diagnosis and treatment. Prog Retin Eye Res 2004; 23:579.
- Fleming TE, Korman NJ. Cicatricial pemphigoid. J Am Acad Dermatol 2000; 43:571.
- Tauber J, Jabbur N, Foster CS. Improved detection of disease progression in ocular cicatricial pemphigoid. Cornea 1992; 11:446.
- Power WJ, Neves RA, Rodriguez A, et al. Increasing the diagnostic yield of conjunctival biopsy in patients with suspected ocular cicatricial pemphigoid. Ophthalmology 1995; 102:1158.
- Sacher C, Hunzelmann N. Cicatricial pemphigoid (mucous membrane pemphigoid): current and emerging therapeutic approaches. Am J Clin Dermatol 2005; 6:93.
- Hall VC, Liesegang TJ, Kostick DA, Lookingbill DP. Ocular mucous membrane pemphigoid and ocular pemphigus vulgaris treated topically with tacrolimus ointment. Arch Dermatol 2003; 139:1083.
- Alonso A, Bignone ML, Brunzini M, Brunzini R. Ocular autoimmune pemphigoid and cyclosporin A. Allergol Immunopathol (Madr) 2006; 34:113.
- Chang JH, McCluskey PJ. Ocular cicatricial pemphigoid: manifestations and management. Curr Allergy Asthma Rep 2005; 5:333.
- Kirtschig G, Murrell D, Wojnarowska F, Khumalo N. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev 2003; :CD004056.
- Miserocchi E, Baltatzis S, Roque MR, et al. The effect of treatment and its related side effects in patients with severe ocular cicatricial pemphigoid. Ophthalmology 2002; 109:111.
- Wertheim MS, Males JJ, Cook SD, Tole DM. Dapsone induced haemolytic anaemia in patients treated for ocular cicatricial pemphigoid. Br J Ophthalmol 2006; 90:516.
- McCluskey P, Chang JH, Singh R, Wakefield D. Methotrexate therapy for ocular cicatricial pemphigoid. Ophthalmology 2004; 111:796.
- Hervás Ontiveros A, Salom D, España Gregori E, et al. Methotrexate as a treatment in ocular cicatricial moderate pemphigoid. Arch Soc Esp Oftalmol 2014; 89:447.
- Saw VP, Dart JK, Rauz S, et al. Immunosuppressive therapy for ocular mucous membrane pemphigoid strategies and outcomes. Ophthalmology 2008; 115:253.
- Nottage JM, Hammersmith KM, Murchison AP, et al. Treatment of mucous membrane pemphigoid with mycophenolate mofetil. Cornea 2013; 32:810.
- Lugović L, Buljan M, Situm M, et al. Unrecognized cicatricial pemphigoid with oral manifestations and ocular complications. A case report. Acta Dermatovenerol Croat 2007; 15:236.
- Dave VK, Vickers CF. Azathioprine in the treatment of muco-cutaneous pemphigoid. Br J Dermatol 1974; 90:183.
- Friedman J, Marcovich AL, Kleinmann G, Schattner A. Low-dose pulsed intravenous cyclophosphamide for severe ocular cicatricial pemphigoid in elderly patients. Cornea 2014; 33:1066.
- Munyangango EM, Le Roux-Villet C, Doan S, et al. Oral cyclophosphamide without corticosteroids to treat mucous membrane pemphigoid. Br J Dermatol 2013; 168:381.
- Perlis C, Pan TD, McDonald CJ. Cytotoxic agents. In: Comprehensive Dermatologic Drug Therapy, 2nd ed, Wolverton SE (Ed), Elsevier, 2007. p.197.
- Letko E, Miserocchi E, Daoud YJ, et al. A nonrandomized comparison of the clinical outcome of ocular involvement in patients with mucous membrane (cicatricial) pemphigoid between conventional immunosuppressive and intravenous immunoglobulin therapies. Clin Immunol 2004; 111:303.
- Foster CS, Ahmed AR. Intravenous immunoglobulin therapy for ocular cicatricial pemphigoid: a preliminary study. Ophthalmology 1999; 106:2136.
- Sami N, Letko E, Androudi S, et al. Intravenous immunoglobulin therapy in patients with ocular-cicatricial pemphigoid: a long-term follow-up. Ophthalmology 2004; 111:1380.
- Letko E, Bhol K, Foster SC, Ahmed RA. Influence of intravenous immunoglobulin therapy on serum levels of anti-beta 4 antibodies in ocular cicatricial pemphigoid. A correlation with disease activity. A preliminary study. Curr Eye Res 2000; 21:646.
- Ross AH, Jaycock P, Cook SD, et al. The use of rituximab in refractory mucous membrane pemphigoid with severe ocular involvement. Br J Ophthalmol 2009; 93:421.
- Foster CS, Chang PY, Ahmed AR. Combination of rituximab and intravenous immunoglobulin for recalcitrant ocular cicatricial pemphigoid: a preliminary report. Ophthalmology 2010; 117:861.
- Le Roux-Villet C, Prost-Squarcioni C, Alexandre M, et al. Rituximab for patients with refractory mucous membrane pemphigoid. Arch Dermatol 2011; 147:843.
- Shetty S, Ahmed AR. Critical analysis of the use of rituximab in mucous membrane pemphigoid: a review of the literature. J Am Acad Dermatol 2013; 68:499.
- Heelan K, Walsh S, Shear NH. Treatment of mucous membrane pemphigoid with rituximab. J Am Acad Dermatol 2013; 69:310.
Faculty Approval by: Mark D. Mifflin, MD Brian E. Zaugg, MD & Colin Ip, MD
Copyright statement: Copyright ©2021. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/