Retinoblastoma
Home / Basic Ophthalmology Review / Vitreous
Title: Retinoblastoma
Author: Spencer Fuller, MSIV – UC San Diego School of Medicine, MPH
Definition: Retinoblastoma (Rb) is an intraocular tumor occurring most commonly during childhood from spontaneous or familial mutations in chromosome 13. It is the most common pediatric ocular tumor (1 in 15,000 live births) and can be either unilateral or bilateral.
Presentation: Rb most commonly presents as leukocoria, or an asymmetric, “white”, dimmed, or otherwise abnormal red reflex, often noticed by parents or caregivers. The white pupillary reflex comes up in photos or can be caught by a clinician on routine direct ophthalmoscopy. Strabismus is also a common presenting sign, and one reason every child needs a dilated eye exam who develops strabismus.
The typical patient with Rb from a spontaneous mutation presents at 24 months of age with a unilateral tumor. On the other hand, the typical patient with familial Rb from germline mutations presents at 12 months or even younger and is more likely to have bilateral involvement. They are also at a significantly increased risk of secondary malignancies such as sarcomas (especially osteosarcomas of long bones) and pinealoblastoma [1]. Historically, patients with bilateral Rb and a pinealoblastoma are said to have developed “trilateral retinoblastoma.”
Differential Diagnosis (of leukocoria):
The differential diagnosis of leukocoria in children is broad and includes numerous hereditary, developmental, inflammatory, and miscellaneous conditions as well as benign and malignant non-Rb tumors [2]. These include, but are not limited to:
- Coats’ disease
- Persistent hyperplastic primary vitreous
- Inflammation (i.e. ocular toxocariasis, congenital CMV, orbital cellulitis)
- Congenital cataract
- Retinopathy of prematurity
- Strabismus causing asymmetric red reflexes
- Retinal detachment
- Other intraocular tumors
Diagnosis: Diagnosis is challenging and unique for intraocular tumors because biopsy and histopathologic diagnosis is not available due to the high risk of seeding the tumor outside of the eye. Thus, the workup includes using several imaging and diagnostic modalities, many of which are done under anesthesia, such as:
- Slit lamp biomicroscopy and indirect ophthalmoscopy
- Retinal fundus photography
- Fluorescein Angiography – Rb is highly vascularized and its arteries fill early in the vascular filling phase
- Orbital/Head imaging to narrow the differential diagnosis and to assess for tumor size, calcification, concurrent retinal detachment, and other intracranial tumors associated with Rb (i.e. pinealoblastoma):
- B-scan ultrasonography
- MRI head and orbits (with high-resolution techniques such as surface coil, gadolinium enhancement, and fat suppression when available. [3])
- CT scanning is avoiding due to the risk radiation exposure, especially to patients who might have a germline Rb mutation.
- Conclusive diagnosis requires pathologic diagnosis and can be obtained via fine-needle aspiration (FNA). However, FNA is contraindicated in most if not all cases due to risk of “seeding” tumor cells via needle trauma. As a result, a conclusive diagnosis is often not made and choice of treatment is usually based on imaging results.
- Genetic testing for the Rb mutation can also provide information regarding whether the tumor is of germline origin and thus clarifies risk for bilateral involvement, secondary tumors at time of presentation, and future malignancies. Patients with the germline mutation require serial eye exams for surveillance of future tumors and well as lifelong serial MRI’s for secondary tumors.
Management: Rb spontaneously regresses in about 5% of cases. The other 95% of cases may require:
- Systemic chemoreduction – used to shrink tumor prior to other treatment modalities in an attempt to avoid retinal detachments, extensive vision loss or surgical removal of the globe (enucleation). Chemotherapy is typically reserved for bilateral cases.
- Intra-arterial and intra-vitreal chemotherapy – newer therapies that minimize systemic exposure to chemotherapeutic agents and often can salvage a globe. Intra-vitreal chemotherapy is particularly useful for tumors with vitreous seeding. The advent of these techniques have dramatically decreased the number of enucleations.
- Transpupillary Thermotherapy, Laser Photocoagulation, or Cryotherapy – direct damage to the tumor and destroys the tumor’s blood supply.
- Plaque radiotherapy – a radioactive plaque that is sutured to the surface of the tumor to provide targeted, localized radiation therapy while sparing the remainder of the retina
- External beam radiation therapy – more of a historic treatment than a current one.
- Enucleation – reserved for large, unilateral cases. Avoided if possible, but often necessary.
Complications:
- Retinal detachment
- Orbital cellulitis
- Metastasis
- Loss of vision
- Enucleation
- Death – still unfortunately common in the developing world due to delayed presentation
IMAGE(S) and/or VIDEO(S):

This patient with leukocoria eventually was diagnosed with Retinoblastoma and underwent enucleation.
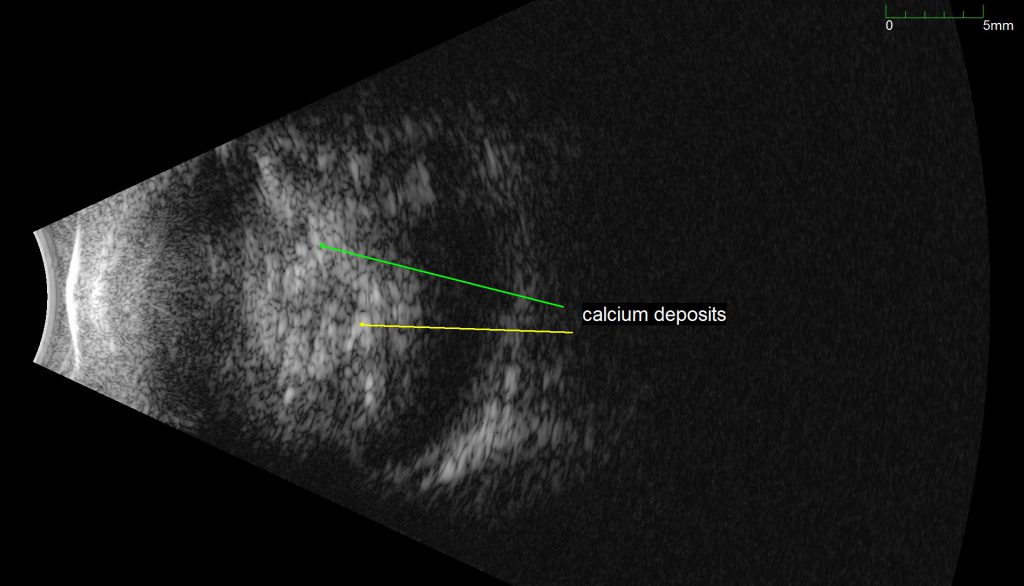
An ultrasound B-scan demonstrating a retinal mass with characteristic calcifications, providing evidence that the tumor is a Retinoblastoma.
Jordan, Michael (2014). 2 year Old with Leukocoria. Moran Eye Center Grand Rounds http://morancore.utah.edu/section-06-pediatric-ophthalmology-and-strabismus/case-2-year-old-with-leukocoria/
REFRENCES:
- Shields, J. A., Shields, C. L. (2008). Retinoblastoma: Introduction, Genetics, Clinical Features, Classification. In Intraocular Tumors: An Atlas and Textbook (pp. 293-318). Lippincott Williams & Wilkins, Philadelphia, PA.
- Stagg, B., Ambati, BK. et al. (2014) Diagnostic Ophthalmology. Amirsys Publishing, Inc., Manitoba, Canada.
- Shields, J. A., Shields, C. L. (2008). Retinoblastoma: Diagnostic Approaches. In Intraocular Tumors: An Atlas and Textbook (pp. 319-326). Lippincott Williams & Wilkins, Philadelphia, PA.
- Shields, J. A., Shields, C. L. (2008). Retinoblastoma: Management of Retinoblastoma. In Intraocular Tumors: An Atlas and Textbook (pp. 327-332). Lippincott Williams & Wilkins, Philadelphia, PA.
- Shields, J. A., Shields, C. L. (2008). Lesions That Can Simulate Retinoblastoma. In Intraocular Tumors: An Atlas and Textbook (pp. 353-366). Lippincott Williams & Wilkins, Philadelphia, PA.
- Kamihara, J., Bourdeaut, F., Foulkes, W. D., Molenaar, J. J., Mossé, Y. P., Nakagawara, A., … & Walsh, M. F. (2017). Retinoblastoma and Neuroblastoma Predisposition and Surveillance. Clinical Cancer Research, 23(13), e98-e106.
- Lohmann DR, Gallie BL. Retinoblastoma. 2000 Jul 18 [Updated 2015 Nov 19]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1452/
Identifier: Moran_CORE_23958




